Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[NA Eun-kyung, Edaily Reporter] South Korean biotech firm Inventage Lab has unveiled promising results from its first oral drug formulation project, achieving a 24.3% bioavailability rate for oral semaglutide, a key obesity drug candidate. The result is 73 times higher than that of Novo Nordisk’s existing oral product, Rybelsus, setting off strong interest across the pharmaceutical industry.
In an interview with Edaily at the company’s Pangyo headquarters on April 24, CEO Joohee Kim said, “Since presenting the data on our oral obesity drug candidate IVL3027 during our investor relations session on April 23, we have received multiple inquiries from global pharmaceutical companies. We initially heard from Novo Nordisk Asia-Pacific, but meetings quickly escalated to include the U.S. division.”
Kim added that Inventage Lab plans to continue initial discussions and participate in major partnering events such as BIO International Convention and the American Diabetes Association (ADA) Scientific Sessions in June to secure licensing deals. “We are building a robust data package to maximize the chances of signing a partnership,” she said.
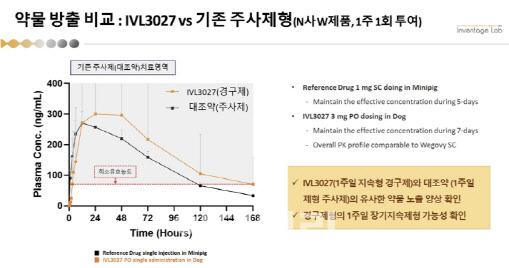 | | In beagle studies, drug exposure patterns for Inventage Lab’s IVL3027 were found to be similar to those of Novo Nordisk’s once-weekly injectable Wegovy, according to the company. (Source: Inventage Lab) |
|
First Oral Drug Attempt Yields Breakthrough Results Inventage Lab, best known for its proprietary IVL-DrugFluidic platform for long-acting injectable formulations, primarily targeted obesity treatments through injectable drugs. The newly unveiled project marks its first attempt at developing an oral formulation using a new platform.
“Semaglutide itself is a potent molecule with a long half-life of about a week, but absorption barriers limit its efficacy and shorten its functional half-life,” Kim said. “Our goal was to preserve the original physical and chemical properties of the active pharmaceutical ingredient (API) without damaging its structure, ensuring full efficacy.”
While Novo Nordisk‘s SNAC (Smart Nutrient Absorption Carrier) technology helps oral semaglutide cross the gastric mucosa, its actual bioavailability remains extremely low―around 0.5% to 1%, compared to injectable formulations like Wegovy. This limited absorption was a key reason Rybelsus gained FDA approval only as a diabetes treatment, not for obesity.
In its preclinical studies, Inventage Lab used beagle dogs―the same model used by Novo Nordisk for Rybelsus development―for a more direct comparison.
According to Kim, “In the Rybelsus NDA, human absorption rates were around 0.5% to 1%, while beagle data showed 0% to 2.7%, depending on dosage. In our case, IVL3027 at 3 milligrams achieved higher bioavailability than 5 milligrams of Rybelsus, with absolute bioavailability exceeding 24%.”
New Oral Platform Ready for Large-Scale Production Inventage Lab’s key platforms include IVL-DrugFluidic for microparticles and IVL-GeneFluidic for nanoparticles. Using the latter, the company encapsulated semaglutide into nanoparticles, significantly enhancing absorption efficiency in the upper small intestine while maintaining the drug’s weeklong half-life.
Kim stressed that Inventage Lab’s in-house contract development and manufacturing organization (CDMO) platform is already capable of mass production. “Based on our IVL-GeneFluidic technology, we have developed a new platform, IVL-PePOFluidic, specialized for oral peptide formulations,” she said. “Since IVL-GeneFluidic was originally designed for large-scale manufacturing, the new PePOFluidic platform inherits that capability. We are currently developing equipment capable of producing enough doses for 1 million patients per batch under a government project.”
 | | Inventage Lab CEO Joohee Kim (Source: Inventage Lab) |
|
The company sees potential applications not just for semaglutide but also for other GLP-1 based drugs such as tirzepatide―the active ingredient in Eli Lilly’s Zepbound―and other peptide candidates under clinical development.
Kim said, “Our immediate focus is applying IVL-PePOFluidic to GLP-1 drugs. As for IVL3027, after optimizing the formulation, we will prepare for toxicity studies and clinical Phase 1 trials. Depending on progress, a technology transfer deal may occur before entering Phase 1.”
Expanding Obesity Drug Pipeline Inventage Lab currently has three major obesity-related drug programs:
A one-month long-acting injectable obesity treatment under joint development with Yuhan Corp.
An ultra-long-acting injectable obesity treatment being co-developed with Boehringer Ingelheim.
The newly announced oral semaglutide project, which Inventage Lab is developing independently.
Kim also shared updates on the Yuhan collaboration. “We originally aimed for 28-day efficacy but confirmed in experiments that the drug maintains efficacy for 42 days. In obesity animal models, we observed both body weight reduction and improved fatty liver outcomes,” she said. “We are now preparing for preclinical studies to submit an Investigational New Drug (IND) application. The Boehringer Ingelheim collaboration is also progressing smoothly as scheduled.”




![2% Royalty Shock at Alteogen Ripples Through Korean Biotech[K-Bio Pulse]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/01/PS26012200181b.jpg)



!['2% 로열티'가 무너뜨린 신뢰…알테오젠發 바이오株 동반 하락[바이오맥짚기]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/01/PS26012201091b.jpg)


