Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[Kim Jin-soo, Edaily Reporter] HLB’s liver cancer drug combination therapy of rivoceranib and camrelizumab has been included as a first-line treatment in the treatment guidelines of the European Society for Medical Oncology (ESMO), the world’s most prestigious oncology society. This news has garnered significant attention in the market.
Following the announcement, not only did the stock prices of HLB(028300), HLB(028300)Pharma, and HLB(028300)LifeScience, which are directly related to the approval of the rivoceranib and camrelizumab combination therapy, rise, but shares of HLB(028300)Therapeutics, a subsidiary of the HLB Group, also surged.
HLB Group Stocks SurgeAccording to KG Zeroin’s MP Doctor (formerly MarketPoint), HLB’s stock price closed at 92,500 won, up 7,900 won (9.34%) from the previous day‘s 84,600 won. At one point in the morning, it even reached 97,500 won. HLB Pharma rose 17.29% to 31,550 won, while HLB Life Science climbed 5.53% to close at 11,250 won.
The sharp rise in HLB Group stocks is attributed to expectations for the approval of rivoceranib, the liver cancer drug under development. HLB holds the global patent for rivoceranib, while HLB Life Science owns the licensing and revenue rights for the Korean, European, and Japanese markets. HLB Pharma holds exclusive sales rights in Korea.
On the same day, HLB announced that its liver cancer drug combination therapy, rivoceranib and camrelizumab, was listed as a first-line treatment in the hepatocellular carcinoma diagnosis and treatment guidelines of ESMO, the world’s leading oncology society. ESMO also issued a “strong recommendation” for prescribing the therapy to liver cancer patients.
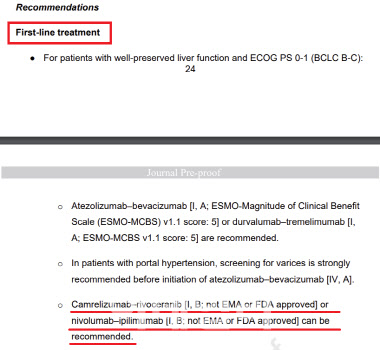 | | ESCO Clinical Practice Guideline - Hepatocellular Carcinoma. (ESCO) |
|
ESMO is considered one of the world’s top three oncology societies alongside the American Society of Clinical Oncology (ASCO) and the American Association for Cancer Research (AACR). The ESMO guidelines for liver cancer provide critical information on diagnosis and treatment, serving as a reference for medical professionals worldwide alongside the guidelines from the National Comprehensive Cancer Network (NCCN).
An HLB representative stated, “To our knowledge, there has never been a case where a drug was listed as a first-line treatment in a society’s guidelines and strongly recommended for prescription before receiving regulatory approval. The announcement of clinical data for the rivoceranib and camrelizumab combination therapy at ESMO in 2022 attracted significant attention and likely influenced this guideline inclusion.”
Typically, medical societies include only approved drugs in their treatment guidelines. While there are numerous cases where off-label prescriptions are recommended for indications not yet secured by approved drugs, it is extremely rare for a drug to be included in guidelines before receiving approval.
However, the inclusion of the rivoceranib and camrelizumab combination therapy in the ESMO guidelines does not guarantee approval from the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA). HLB is currently awaiting the FDA’s decision on the combination therapy, with results expected by March 20.
An HLB representative emphasized, “The inclusion in the guidelines and the strong recommendation signify recognition of the drug’s efficacy, but regulatory approval is a separate matter, so we cannot definitively say that approval is certain.”
Nonetheless, when seeking approval in Europe, the inclusion in ESMO guidelines may provide indirect support. HLB plans to submit its EMA approval application for the rivoceranib and camrelizumab combination therapy as early as the third quarter of this year, or at the latest, by the end of the year.
“Since the combination therapy was conducted as a global clinical trial, there is no need for additional clinical trials for EMA approval,” an HLB representative explained. “If FDA approval is granted, EMA approval is expected to follow more smoothly.”
According to Horizon Grand View Research, the European liver cancer drug market was valued at $1.09 billion (1.6 trillion won) last year. It is projected to grow at a compound annual growth rate (CAGR) of 17.8% from 2025 to 2030, reaching approximately $1.095 billion (3 trillion won).
Oscotec Sees Sixfold Revenue Increase Amid Lecraza GrowthMeanwhile, Oscotec’s stock price rose 11.65% to 29,700 won on the same day. This increase is attributed to strong earnings performance from the previous year.
On January 26, Oscotec announced that its consolidated sales reached 34.1 billion won, a 587% increase from 5 billion won in the same period the previous year. During this period, operating losses were 2.7 billion won, narrowing by 30 billion won year over year. On a standalone basis, the company turned a profit, signaling a strong performance.
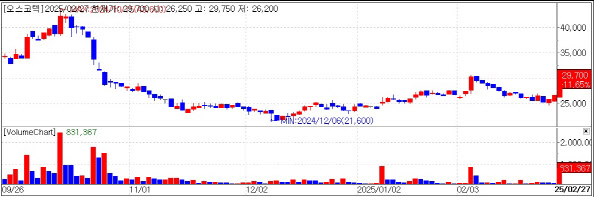 | | Oscotec Stock price.(KG Zeroin’s MP Doctor) |
|
Oscotec’s positive results are largely driven by technology fees and royalties from Lecraza, developed in collaboration with Yuhan Corporation. Oscotec is also expected to secure additional milestone payments following the European Commission (EC) approval of Lecraza. The milestone payment is estimated to be around 17 billion won.
An Oscotec representative noted, “Foreign investors purchased approximately 150,000 shares, and institutions, including pension funds, bought 180,000 shares. This indicates their confidence in Oscotec’s value.”
A variable in Oscotec’s valuation is the planned listing of its subsidiary, Genosco. Oscotec’s shareholder coalition filed a lawsuit on February 14, arguing that Genosco’s IPO would reduce Oscotec’s corporate value, ultimately harming shareholders.
An Oscotec representative countered, stating, “Oscotec is not directly involved in Genosco’s IPO process. The listing and growth of Genosco will, in fact, enhance Oscotec’s value.”




![i-Sens Soars 23% on EU CGM Expansion [K-bio pulse]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/02/PS26021300327b.jpg)



![냉탕 온탕 오간 에이프릴바이오…실적 호조에 로킷·휴젤 상승[바이오맥짚기]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/02/PS26021200275b.jpg)
![[부고]서홍민(엠투엔그룹 회장)씨 모친상](https://image.edaily.co.kr/images/content/defaultimg.jpg)

