Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[Shin Min-joon, Edaily Reporter] On the 4th the stock prices of Voronoi, Yuhan Corporation, and Kolon TissueGene showed a noticeable rise in the South Korean pharmaceutical and bio stock market. The increase in Voronoi and Yuhan Corporation’s stock prices is analyzed to reflect expectations for their new drug development in non-small cell lung cancer and allergic diseases, respectively. Kolon TissueGene’s stock price surged due to expectations that the export of its osteoarthritis treatment TG-C (Invossa) to the U.S. will gain momentum.
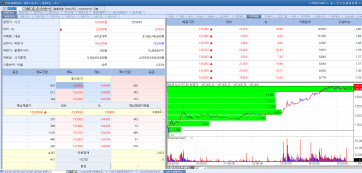 | | Voronoi stock info. on Mar 4. (Image=MP Doctor) |
|
Voronoi, Announces First Clinical Results for Non-Small Cell Lung Cancer DrugAccording to KG Zeroin MP Doctor Voronoi’s stock price rose 20.86% from the previous day to 135,000 won. The rapid progress of the clinical trial for VRN11, one of Voronoi’s leading new drug pipelines for non-small cell lung cancer treatment, has positively influenced the stock price, as the results of Phase 1a were disclosed earlier than expected. Voronoi plans to present the interim results of VRN11’s Phase 1a trial at the American Association for Cancer Research (AACR) conference in the U.S. at the end of next month.
Earlier, Voronoi had announced in its corporate presentation update in early February that it planned to disclose the interim results of VRN11’s Phase 1a trial at the AACR conference in late May. VRN11 is a fourth-generation EGFR-targeted therapy for non-small cell lung cancer developed by Voronoi. According to the company, VRN11 has superior efficacy compared to existing drugs for EGFR Del19, L858R, and their compound mutations. Since March 2024, Voronoi has been conducting a dose-escalation trial for Phase 1a in Korea and Taiwan on patients who are unresponsive or have progressed after available standard therapies, including tyrosine kinase inhibitors (TKI).
VRN11 has demonstrated excellent efficacy against various EGFR mutations, including acquired resistance mutations such as EGFR C797S, as well as common mutations like EGFR Del19 and L858R. In monkey trials, VRN11 showed a brain-blood barrier penetration rate of over 100%, making it a potential treatment for brain metastasis patients. Voronoi has also secured strong safety data from GLP non-clinical toxicity tests and is conducting aggressive dose escalation trials (100-100-100-100-50% escalation).
To accelerate VRN11’s clinical development, Voronoi has expanded the scale of its Phase 1 trial from 50 to 103 patients through two protocol amendments, which were approved by the Ministry of Food and Drug Safety. Meanwhile, the research on VRN07 (ORIC114), a treatment for EGFR exon 20 deletion mutations in non-small cell lung cancer that Voronoi licensed to Oric Pharmaceuticals in the U.S. is progressing smoothly.
A combination clinical trial of ORIC114 with Janssen’s non-small cell lung cancer treatment, Rybrevant, is expected to commence soon. While VRN11 targets the common EGFR mutations Del19 and L858R, which account for 80-90% of primary mutations, VRN07 specifically targets the less common EGFR exon 20 deletion mutations, which make up 10-20%.
Voronoi is also developing VRN10, a HER2-positive solid tumor treatment. The company has applied for a Phase 1 clinical trial to evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics, and efficacy of VRN10 in HER2-positive and HER2-mutant solid tumor patients. This Phase 1 trial is expected to be conducted in five institutions across Korea and Australia, targeting around 70 patients, including those with HER2-positive breast cancer.
A Voronoi representative stated “VRN10 has high selectivity for HER2 and also exhibits strong activity against HER2 solid tumors resistant to Enhertu, making it a promising new treatment option for patients.”
Yuhan Corporation’s Allergy Drug Shows Improvement in Chronic Urticaria SymptomsYuhan Corporation‘s stock price rose 6.48% from the previous day, closing at 129,800won. The increase is attributed to positive clinical trial results showing that Yuhan’s investigational allergy treatment, Resigercept (YH35324), may improve symptoms in patients with chronic urticaria who do not respond to antihistamines. Resigercept demonstrated favorable results compared to omalizumab (brand name Xolair), the existing standard treatment, in inhibiting free serum immunoglobulin E (IgE).
Yuhan Corporation recently presented the Phase 1b Part 1 clinical trial results for Resigercept in a poster session at the 2025 annual meeting of the American Academy of Allergy, Asthma & Immunology (AAAAI). Resigercept is an Fc fusion protein-based anti-immunoglobulin E (anti-IgE) drug that reduces IgE levels to alleviate allergic symptoms.
The Phase 1b trial was conducted at nine university hospitals in Korea, with results for Part 1 announced at the conference. The study aimed to evaluate the effects of a single subcutaneous injection of Resigercept as an add-on therapy to H1 antihistamines in patients with chronic spontaneous urticaria unresponsive to antihistamines over an eight-week period. The trial confirmed Resigercept‘s safety, and it exhibited strong and sustained suppression of free IgE levels compared to omalizumab.
Using UAS7 (a seven-day urticaria activity score) as a clinical metric, the study found that the proportion of patients achieving complete symptom resolution (UAS7 score of 0) was higher in the 6 mg/kg Resigercept group than in the omalizumab group.
A Yuhan Corporation representative stated “The trial results confirmed that Resigercept improves clinical symptoms in patients with chronic spontaneous urticaria who are unresponsive to H1 antihistamines, the drug’s primary target indication. We are analyzing the final results of the Phase 1b study, which assesses safety, pharmacokinetics, and pharmacodynamics with repeated dosing, and are reviewing the optimal strategy for the next phase of development.”
Kolon TissueGene’s TG-C Expected to Accelerate U.S. Market EntryKolon TissueGene’s stock price rose 7.3% from the previous day to 38,950 won. Investors anticipate that the company’s osteoarthritis treatment, TG-C (Invossa), will accelerate its entry into the U.S. market, especially after the recent appointment of former Daewoong Pharmaceutical CEO Seung-Ho Jeon as the head of Kolon Group‘s bio-healthcare division and CEO of Kolon TissueGene.
Jeon who served as CEO of Daewoong Pharmaceutical from 2018 to 2024, is credited with significantly contributing to the company’s achievement of 1 trillion KRW in annual sales. He will concurrently oversee Kolon Group’s bio-healthcare division and serve as co-CEO of Kolon TissueGene alongside Jong-Moon Noh. The group had previously been criticized for lacking a control tower to oversee its bio-healthcare subsidiaries, which include Kolon Pharmaceuticals, Kolon Life Science, and Kolon TissueGene.
Kolon TissueGene is currently conducting Phase 3 clinical trials for TG-C in the U.S., having completed patient administration for 1066 individuals across 80 clinical sites last year. The company aims to obtain U.S. FDA Biologics License Application (BLA) approval for TG-C by 2027. Kolon TissueGene holds the U.S. and European rights for TG-C, while its affiliate Kolon Life Science owns the rights for Korea and Greater China (China, Hong Kong, Macau, and Taiwan). Juniper Therapeutics, a Singapore-based company, holds the rights for Asia, the Middle East, and Africa.
A Kolon TissueGene representative stated “The U.S. Phase 3 trial for TG-C is progressing smoothly, and we are committed to completing it as scheduled.”



![2% Royalty Shock at Alteogen Ripples Through Korean Biotech[K-Bio Pulse]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/01/PS26012200181b.jpg)



!['2% 로열티'가 무너뜨린 신뢰…알테오젠發 바이오株 동반 하락[바이오맥짚기]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/01/PS26012201091b.jpg)


