Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[Song Young Doo, Edaily Reporter] Shaperon shares hit the upper price limit after its preclinical study on an alopecia areata therapy demonstrated efficacy. Hans Biomed also rallied sharply on expectations following the early launch of its next-generation skin booster. Neurophet, recently named one of the world’s top health-tech companies by TIME, gained further as it obtained Medical Device Single Audit Program(MDSAP) certification, raising hopes for faster regulatory approvals.
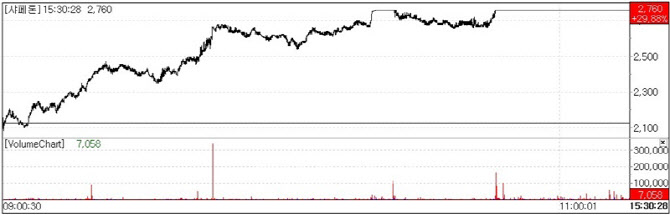 | | Stock trend of Shaperon.(Source : KG Zeroin MP Doctor) |
|
On the 24th, according to KG Zeroin’s MP Doctor(formerly Market Point), Shaperon closed at 2,760 won, up 29.88%(635 won) from the previous session. The rally was attributed to news that the company confirmed efficacy of its alopecia areata therapy in preclinical trials.
Shaperon announced it presented the results of preclinical studies on SH1010337, its alopecia areata candidate, at the European Academy of Dermatology and Venereology(EADV). The data confirmed hair growth promotion through immune modulation. Based on years of GPCR19 research, the company said it has introduced the world’s first immune control based therapy for alopecia areata. SH1010337 suppressed inflammasome overactivation and increased regulatory T cells(Tregs), thereby normalizing scalp immunity and promoting follicle regeneration in an autoimmune alopecia model.
In the LNC(lymph node cell induced) mouse model, a standard preclinical model for alopecia areata, SH1010337 significantly reduced bald patches. While the JAK inhibitor comparator showed a recovery rate of around 62%, SH1010337 achieved 74%, confirming superior efficacy. Analysts noted that unlike existing therapies, SH1010337 showed stronger immune modulation and hair regrowth without the broad immunosuppression and relapse risks commonly associated with JAK inhibitors. The results suggest differentiated competitiveness in restoring immune balance and ensuring long-term safety.
While global pharmaceutical firms have previously failed to develop GPCR19-targeting therapies, Shaperon emphasized its differentiated success. The company said, “Through our AI platform AIDEN, trained on years of big data, we achieved breakthroughs in drug design and toxicity prediction that traditional algorithms struggled to deliver.”
Shaperon added, “As the industry’s first GPCR19-targeting alopecia therapy, SH1010337 is viewed as a potential game changer. It is an innovative compound derived from more than a decade of accumulated inflammasome research and AI learning.”
A company official noted, “This preclinical study not only demonstrated efficacy in alopecia areata but also showed potential for broader application to autoimmune diseases. Since SH1010337 is based on NuGel, our topical atopic dermatitis therapy that has proven both effective and safe in U.S. and Korean Phase II trials, we expect a strong human safety profile.”
Hans Biomed Jumps on Early Launch of Skin Booster Hans Biomed also rose sharply, ending the day at 18,100 won, up 20.67%(3,100 won). The stock has more than doubled over the past 10 months, climbing from 9,060 won on January 2.
The rally is attributed to anticipation surrounding the early launch of its new growth engine, the skin booster “Cellerdiem.” Originally scheduled for official release on the 29th, the company advanced the launch to the 22nd for strategic reasons. PharmEdaily recently published an interview with CEO Kim Geun-young, in which he outlined the firm’s skin booster strategy, further fueling investor optimism.
Unlike conventional boosters that merely stimulate collagen production by improving the dermal environment, Cellerdiem directly replenishes collagen using human acellular dermal matrix(hADM). Designed as 75μm ultra-fine particles, the product delivers collagen more abundantly and uniformly.
In the interview, Kim said, “Compared to existing PN/PDRN boosters dominating the market, Cellerdiem demonstrates superior skin improvement. Its particles are 25% smaller than earlier ECM boosters, reducing injection pain and improving absorption and retention. Since we market it directly through our subsidiary Mint Medical, distribution costs are minimal, ensuring high margins.”
He added, “Considering competitor sales, we expect initial monthly sales of over 5,000 units. Within a year, once the product is established, annual domestic sales could exceed 20 billion won, with rapid growth expected as we expand into China.”
Neurophet Gains on Regulatory Pathway Acceleration Neurophet, which spiked on the 22nd after being named a top global health-tech company by TIME, continued its rally, closing at 22,050 won, up 11.53%(2,280 won). The stock has more than doubled in September, surging 114% from 10,300 won on the 1st to the 24th.
On the 23rd, Neurophet announced it had obtained MDSAP certification, a program led by the International Medical Device Regulators Forum(IMDRF) and recognized by the U.S., Canada, Japan, Australia, and Brazil. MDSAP allows a single audit to certify compliance with safety and quality standards across multiple jurisdictions.
With certification valid for three years, Neurophet can streamline product approvals and reduce both time and cost, as national regulators like the U.S. FDA may waive some or all on-site inspections. A company representative said, “Today’s rally reflects optimism over the MDSAP announcement. Since the certification can replace multiple country-level audits, especially in the U.S. and Japan, investors expect accelerated global expansion.”



![2% Royalty Shock at Alteogen Ripples Through Korean Biotech[K-Bio Pulse]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/01/PS26012200181b.jpg)



!['2% 로열티'가 무너뜨린 신뢰…알테오젠發 바이오株 동반 하락[바이오맥짚기]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/01/PS26012201091b.jpg)


