Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[Song Young Doo, Edaily Reporter] Domestic biopharma stocks surged on overseas catalysts. D&D Pharmatech hit the daily upper limit after reports that its obesity drug partner Mesera is being eyed by Pfizer. Neurophet also rallied sharply after being named one of the world’s top healthtech companies by TIME.
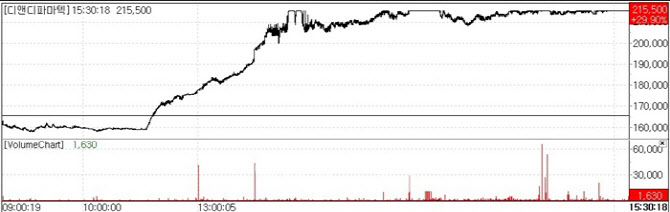 | | Stock trend of D&D Pharmatech.(Source : KG Zeroin MP Doctor) |
|
According to KG Zeroin’s MP Doctor(formerly Market Point), shares of D&D Pharmatech closed at 215,500, up 29.90% from the previous day. The stock initially traded weaker but reversed course around 1 p.m. and surged into the close.
The rally came after the Financial Times and Reuters reported that Pfizer is considering acquiring Mesera, a U.S. biotech developing obesity therapies, in a deal worth $7.3 billion. D&D Pharmatech had previously out-licensed its oral delivery platform Oralink to Mesera in 2023 and 2024 under deals valued at up to $700 million. If Pfizer completes the acquisition, industry observers say the licensing agreements would transfer to Pfizer.
A D&D Pharmatech official noted: “If the acquisition is finalized, our technology transfer contracts would move from Mesera to Pfizer. Investors see significance in the fact that our obesity drug partner could become Pfizer, the biggest player in the field.”
TIME Names Neurophet Among World’s Top HealthTech Firms Neurophet ended the day up 21.52% at 20,100, its highest level since listing on July 25 at 14,000. The stock’s surge followed news that the company was included in TIME’s inaugural list of the World’s Top HealthTech Companies, announced Sept. 18.
A total of 495 companies worldwide were recognized, including eight from Korea: AITRICS, Medipixel, and EZCaretech in AI and data analytics; Neurophet in diagnostics; Health Connect in telemedicine; Senakle Soft and Kakao Healthcare in health information; and Olive Healthcare in medical devices and wearables.
Of the group, Neurophet and EZCaretech are listed companies, both posting gains. Neurophet shares jumped more than 20%, while EZCaretech rose 1.85% to close at \17,090.
Analysts said inclusion in the TIME ranking enhances brand recognition globally and may accelerate partnerships with multinational companies. Neurophet stated: “We did not apply for the recognition. Being chosen based on TIME’s criteria validates our AI diagnostic technology and drove today’s share price rally.”
TIME’s methodology considered financial performance, reputation analysis, and online engagement, with a focus on companies offering AI, automation, and data-driven diagnostic innovations, including advanced imaging and early disease detection solutions.
Neurophet specializes in AI-based brain imaging analytics for neurological disorders and Alzheimer’s therapeutics. The company recently launched Neurophet AQUA AD, a comprehensive imaging solution for Alzheimer’s drug prescription and treatment efficacy analysis. It is also collaborating with Eli Lilly, training its AI software with PET clinical data since last year.
DAEHWA Pharmaceutical Wins China Nod for Alzheimer’s Patch DAEHWA Pharmaceutical also surged, closing up 26.60% at 18,230, after announcing Chinese approval of its Alzheimer’s rivastigmine transdermal patch. Through its joint venture JHK Biopharm, the company will market the product in China, where dementia patients exceed 10 million, a figure expected to quadruple by 2050.
Branded as Rivamensa Patch, the therapy delivers rivastigmine through a 24-hour transdermal system in 4.6mg and 9mg doses. Industry officials note this marks the first Chinese approval of a Korean transdermal drug delivery system(TDDS) patch.
DAEHWA said it plans to expand its TDDS portfolio into broader Asian and global markets, though specific launch timing and revenue expectations have yet to be determined. A company representative emphasized: “This approval demonstrates that our TDDS technology and product quality have been validated by Chinese regulators.”



![i-Sens Soars 23% on EU CGM Expansion [K-bio pulse]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/02/PS26021300327b.jpg)



![냉탕 온탕 오간 에이프릴바이오…실적 호조에 로킷·휴젤 상승[바이오맥짚기]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/02/PS26021200275b.jpg)


