Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[Kim Jinsoo, Edaily Reporter] On Aug. 20, despite pressure from a downturn in U.S. markets, South Korea’s DXVX and Ildong Pharmaceutical posted sharp gains, drawing investor attention. DXVX climbed on expectations of further licensing-out deals after recent successful technology exports, while Ildong gained momentum as a rising player in the obesity drug sector.
In contrast, Bridge Biotherapeutics fell nearly 5% after announcing it would voluntarily halt development of one of its pipelines, effectively ending further research on that program.
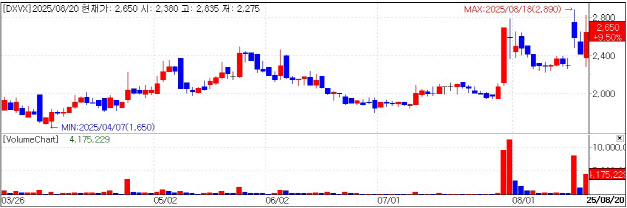 | | DXVX stock trend. (KG Zeroin MP Doctor) |
|
DXVX Eyes Additional Export Deals DXVX shares rose as much as 17.15% intraday to 2,830 won before closing at 2,650 won, up 9.50%. Since July 29, the stock has surged 35.48%. Analysts attributed the rally to back-to-back licensing-out contracts and heightened expectations for more.
In July, DXVX signed its first export contract with a U.S. biotech worth about 300 billion won ($220 million) for co-developing an mRNA-based cancer vaccine. Under the deal, DXVX will receive milestone payments totaling 300 billion won and collect royalties of more than 10% of sales for at least 15 years after commercialization. DXVX will oversee preclinical, clinical, and manufacturing stages, while its partner handles global regulatory approvals and sales. The deal also highlighted DXVX’s long-term storage platform technology.
On Aug. 18, DXVX’s subsidiary Avixgen sealed a separate deal with a U.S. biotech to export its Advanced Cell Penetration Peptide (ACP) drug delivery platform, valued at roughly 500 billion won ($370 million). DXVX, which owns 66.2% of Avixgen, led negotiations on the company’s behalf.
DXVX expects the back-to-back contracts to open doors for additional licensing-out opportunities. CEO Kwon Kyu-chan has set a goal of three to four export deals this year, leaving room for one or two more by year-end. Because platform technologies can be licensed on a nonexclusive basis, more deals could follow.
“We are in discussions with domestic and international pharma companies and are working to finalize another technology export this year,” a company official said.
Ildong Pharma Boosted by Obesity Drug Pipeline Ildong Pharmaceutical closed 7.04% higher at 22,800 won. The stock has been climbing since early July, when it traded at 12,670 won. The rise is tied to its wholly owned subsidiary Unovia and its experimental obesity treatment, ID110521156.
Unovia specializes in new drug development for Ildong. Unlike existing peptide-based GLP-1 therapies that require injection, ID110521156 is a small-molecule, oral GLP-1 receptor agonist. Ildong presented interim Phase 1 results at the American Diabetes Association’s annual meeting in June. Topline Phase 1 results are expected by the end of August.
The candidate showed promising efficacy. In the 100 mg cohort, patients lost an average of 6.9% of body weight over four weeks, with a maximum reduction of 11.9%. No serious adverse events or discontinuations were reported.
“ID110521156 is nearing the end of multiple-ascending-dose Phase 1 testing, and we plan to pursue licensing as part of our commercialization strategy,” an Ildong official said.
Bridge Biotherapeutics Scales Back Bridge Biotherapeutics shares fell 4.85% to 2,450 won after the company said it was voluntarily withdrawing trials of its non-small cell lung cancer drug candidate BBT-207 in both South Korea and the United States. With that move, along with the earlier halts of BBT-877 and BBT-401, the company is effectively shutting down its R&D pipeline.
An official said BBT-207 had already met goals in Phase 1 by showing partial responses and was therefore ready for licensing-out discussions, eliminating the need for further trials.
The decision also reflects a management shift. In June, the company’s largest shareholder changed to Parataxis Korea Fund No. 1, affiliated with a U.S.-based digital asset investment firm. Bridge Biotherapeutics has since pivoted toward crypto assets, disclosing on Aug. 18 the purchase of 14.696 bitcoin for 2.34 billion won ($17 million) through Wavebridge Hong Kong.
The company said the move was intended to preserve long-term asset value and enhance financial flexibility.






![알테오젠, 일시적 투심하락 관망세 전환…삼양바이오팜 가파른 상승[바이오맥짚기]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/01/PS26012300239b.jpg)


