Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[Kim Jinsoo, Edaily Reporter] Shares of artificial-intelligence health care company Noul surged to the daily limit Tuesday on reports it is close to signing large overseas contracts, fueling expectations for stronger results. Peer Neurophet jumped about 10% after its flagship product was designated an “innovative medical technology,” unlocking a path to government support.
Obesity-drug names Hanmi Pharmaceutical and Ildong Pharmaceutical also rallied on optimism tied to Hanmi’s presence at the European Association for the Study of Diabetes meeting and Ildong’s upcoming Phase 1 topline data release.
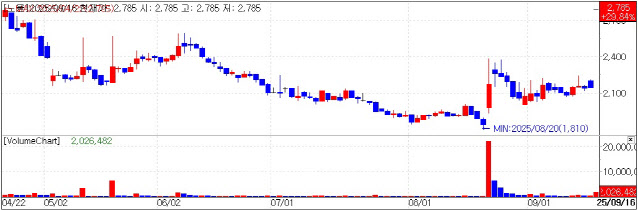 | | Noul Stock Trend. (KG Zeroin MP Doctor) |
|
Noul hits limit-up on imminent supply deals Market tracker KG Zeroin MP Doctor said Noul went straight to limit-up from the open and closed at 2785 won, up 640 won from Monday. The move followed reports that the company expects to finalize large supply agreements in North America and Europe before year-end.
On Sept. 15, Noul said it is close to a supply contract for its blood-analysis product with a leading U.S. firm that controls about 30% of the domestic diagnostics market. It is also reportedly preparing to sign a deal this year with a top European health care company to supply a cervical-cancer diagnostic product. Noul has built traction in Africa with AI malaria diagnostics and now aims to compete in advanced markets with higher value-added products.
The company plans to launch miLab CER, billed as the world’s only one-stop AI cervical-cancer diagnostic, next month in Europe and Latin America, followed by another one-stop AI product in December that enables disease diagnosis from a simple finger-stick blood sample.
Noul also said Sept. 15 it obtained marketing authorization in Vietnam for miLab CER. According to the International Agency for Research on Cancer under the WHO, about 4,600 Vietnamese women are newly diagnosed with cervical cancer each year, and roughly half die from the disease.
Roughly the size of a small printer, Noul’s automated AI diagnostic platform is suited to small and midsize hospitals and to low-income countries. It has drawn attention from international health organizations, including the Bill & Melinda Gates Foundation. Each unit is priced in the tens of millions of won. Noul plans to sell more than 2000 AI diagnostic units globally over the next three years and is expanding factory capacity to meet anticipated demand after North American and European deals are signed.
First-half 2025 revenue came in at 2.8 billion won, already topping full-year 2024 sales of 1.6 billion won. The company targets 6.9 billion won in revenue this year. Operating losses narrowed in the first half after a 22.8 billion won loss last year.
“The stock appears to have jumped on news of pending large supply contracts in North America and Europe and on the Vietnam approval,” a Noul official said.
Neurophet secures second ‘innovative medical technology’ nod Neurophet rose 9.7% to close at 16510 won. The gain followed the designation of Neurophet AQUA AD Plus, its brain-image detection and diagnostic-support software, as an innovative medical technology after an integrated review conducted by the Ministry of Food and Drug Safety and related agencies.
The integrated review and assessment program is designed to speed adoption of medical devices recognized as innovative. With the decision, AQUA AD Plus’s core feature its AI-based cerebral microbleed detection can enter the non-reimbursed or reimbursed market for three to five years.
AQUA AD Plus is an upgraded version of Neurophet AQUA AD, a comprehensive analysis solution used for Alzheimer’s therapy decisions. While AQUA AD focuses on quantitative analysis integrating MRI and PET images, AQUA AD Plus uses AI to analyze brain MRI and automatically detect the location of suspected microbleeds, helping clinicians craft more precise treatment plans. The product was recognized for both technological innovation and clinical utility.
“The AQUA AD Plus technology is expected to be used in clinical settings within the year,” a Neurophet official said, adding the company will continue developing tools to support more precise care.
In June, Neurophet also won an innovative-medical-technology designation for its personalized, AI-guided transcranial direct-current stimulation approach.
Obesity plays Hanmi, Ildong extend gains Hanmi Pharmaceutical climbed 7.02% to 320000 won, while Ildong Pharmaceutical advanced 13.35% to 25900 won. Both have gained on rising interest in obesity treatments.
At EASD 2025, Sept. 15~19 in Vienna, Hanmi is unveiling six preclinical presentations, including one oral, on next-generation candidates: the triple agonist HM15275 (LA-GLP/GIP/GCG), the novel HM17321 (LA-UCN2), and the oral agent HM101460. Notably, Hanmi is presenting data supporting the clinical potential of HM17321 in an obese non-human primate model, along with molecular-level evidence that HM17321 directly promotes muscle mass gain and data on glucose control via metabolic adaptation. Details will be delivered in an oral presentation by Jae-Min Jeon, executive director and head of clinical translation at Hanmi R&D Center.
“Following our showing at the American Diabetes Association in June, we expect EASD to reaffirm the differentiated R&D competitiveness of Hanmi’s next-generation obesity pipeline, and we will accelerate global expansion,” a company official said.
Separately, Unovia, an Ildong Pharmaceutical subsidiary, is slated to report topline Phase 1 results on Sept. 29 for ID110521156, an oral GLP-1-class obesity drug candidate. The update will cover efficacy, safety and development plans.
ID110521156 is a once-daily oral tablet similar to orforglipron, which Eli Lilly has advanced into Phase 3. Interim data released in June showed up to 11.9% weight loss over four weeks in the 100㎎ cohort. With higher-dose cohorts now included, investors expect clearer visibility on the program’s prospects.
“Ildong will pursue parallel strategies of clinical development and technology out-licensing toward commercialization,” a company official said.



![Alteogen Sees Temporary Dip in Investor Sentiment Market Turns Cautious[K-Bio Pulse]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/01/PS26012300236b.jpg)


![알테오젠, 일시적 투심하락 관망세 전환…삼양바이오팜 가파른 상승[바이오맥짚기]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/01/PS26012300239b.jpg)