Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[Kim Saemi, Edaily Reporter] On July 17, shares of Yuhan Corporation and its preferred shares surged on the KOSPI market. HYUNDAI PHARMACEUTICAL CO., LTD. (HYUNDAI PHARM) also jumped 25.36% after being selected for a national drug development project. On the KOSDAQ market, KUKJEON PHARMACEUTICAL Co., Ltd(KUKJEON) and Genexine hit their respective daily upper limits.
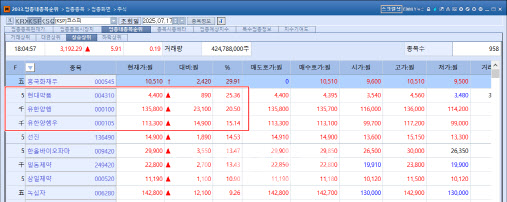 | | On July 17, Hyundai Pharma, Yuhan Corporation, and Yuhan Preferred Shares ranked among the top gainers on the KOSPI market. (Source: KG Zeroin MP Doctor) |
|
Yuhan Surges on Blockbuster Potential of Leclaza According to KG Zeroin’s MP Doctor(formerly Market Point), Yuhan Corporation closed at ₩135,800, soaring ₩23,100 (20.5%) from the previous session. Over the same period, Yuhan’s preferred shares also gained ₩14,900 (15.14%), ending at ₩113,300.
This sharp rally in both Yuhan’s common and preferred shares was largely driven by Johnson & Johnson’s (J&J) second-quarter earnings release on July 16 (local time), which revealed that global sales of the Leclaza (Lazertinib) + Rybrevant combination therapy reached $179 million (approximately ₩248.3 billion) in the second quarter of this year-a 159% increase compared to $69 million in the same period last year.
First-half 2025 sales totaled $320 million (approx. ₩443.8 billion), doubling from a year earlier, with 79% ($252 million) generated in the U.S. market.
This suggests the combination therapy is rapidly becoming a first-line treatment for non-small cell lung cancer (NSCLC). Although the sales breakdown for Leclaza alone was not disclosed, Yuhan receives royalties under its license agreement with J&J.
Joaquin Duato, CEO of J&J, remarked during the earnings call, “The Rybrevant and lazertinib combination is establishing a new standard of care in NSCLC and is becoming a key growth driver within our oncology portfolio.”
Hyundai Pharm Surges on Selection for National Drug Development Project Hyundai Pharm closed at ₩4,400, up ₩890 (25.36%), ranking as the second-highest gainer on the KOSPI.
The stock rallied after news broke around noon that Hyundai’s next-generation anticancer agent, a USP1 inhibitor, had been selected for a government-led drug R&D ecosystem project under the National New Drug Development Project.
The USP1 inhibitor is an innovative synthetic lethality-based anticancer agent that selectively induces cancer cell death by blocking DNA damage repair mechanisms.
Hyundai stated that the selection reflects recognition of its research achievements and expects this to boost its global competitiveness in the synthetic lethality-based oncology drug market.
A company spokesperson commented, “We’ve proven the innovation of our USP1 inhibitor through years of accumulated expertise and talent. With this national project backing, we’ll accelerate R&D to maximize its potential as a global new drug.”
Why Did KUKJEON and Genexine Hit Their Upper Limits? On the KOSDAQ market, KUKJEON and Genexine both hit their respective daily upper limits. KUKJEON surged ₩1,080 (30%) to close at ₩4,680, while Genexine jumped ₩1,520 (29.98%) to ₩6,590.
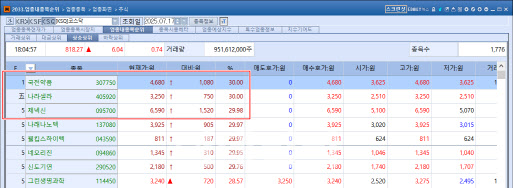 | | On July 17, two out of the seven stocks that hit the daily upper limit on the KOSDAQ market were pharmaceutical and biotech companies. (Source: KG Zeroin MP Doctor) |
|
KUKJEON’s sharp rise appears to have been fueled by news that its subsidiary, KS Biologics (KSBL), signed a supply and license agreement with Kalbe, signaling the company’s official entry into the Southeast Asian market.
According to KUKJEON, Kalbe Group is the largest pharmaceutical company in Southeast Asia, headquartered in Indonesia, with a market capitalization of around ₩7 trillion and annual sales of ₩2.7 trillion last year. KSBL is a contract development and manufacturing organization (CDMO) specializing in oncology drugs, established in 2023 as a joint venture between KUKJEON, a Korean active pharmaceutical ingredient (API) company, and SN Bioscience, a new drug developer.
Under the agreement, Kalbe will handle regulatory approvals, sales, and marketing of KSBL’s oncology pipeline in various Southeast Asian countries. The two companies also plan to maintain a strategic partnership to expand sales in the region.
KSBL is also preparing to enter advanced markets, including Japan, Europe, and the United States, and is currently working on regulatory submissions. Additionally, it is in discussions with global pharmaceutical companies for potential partnerships.
A KUKJEON official stated, “We expect sales from our anticancer products as we enter the Southeast Asian market. With strong interest in our oncology portfolio, we anticipate a smooth expansion of our global presence.”
Genexine’s stock hit the upper limit following the announcement that Chief R&D Officer Jae-Hyun Choi has been invited to speak at the “5th Annual mRNA-Based Therapeutics Summit 2025” to be held on July 22 (local time) in Boston, USA. At the event, he will unveil the development progress of Genexine’s targeted protein degrader (TPD) platform, EPDeg bioPROTAC.
EPDeg bioPROTAC is a structure that directly fuses a synthetic nanobody-based target protein binder with an E3 ligase. The company expects this structure to overcome the limitations of conventional small molecule-based PROTACs.
During the event, Dr. Choi will deliver a presentation titled “Recent Progress in mRNA-Based Targeted Protein Degrader Development,” highlighting the strengths of Genexine’s platform technology and its future direction. He will also introduce the preclinical development results of the company’s leading bioPROTAC candidates, GX-BP1 and GX-BP2.
A Genexine representative stated, “Being invited to give an oral presentation at the world’s leading mRNA therapeutics conference indicates a high level of global interest in Genexine’s bioPROTAC technology,” and added, “We hope this will serve as an opportunity to explore potential co-development and technology licensing partnerships with global players.”




![Alteogen Sees Temporary Dip in Investor Sentiment Market Turns Cautious[K-Bio Pulse]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/01/PS26012300236b.jpg)


![알테오젠, 일시적 투심하락 관망세 전환…삼양바이오팜 가파른 상승[바이오맥짚기]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/01/PS26012300239b.jpg)


