Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[Song Young Doo, Edaily Reporter] ABPROBIO’s preclinical research on a multi-specific antibody drug under co-development with Celltrion was selected for an oral presentation at a U.S. academic meeting. With antitumor efficacy and safety confirmed in the study, ABPROBIO’s share price hit the daily upper limit. Ildong Holdings and Ildong Pharmaceutical rallied on expectations for a potential out-licensing deal for an oral small-molecule GLP-1 RA obesity therapy. Syntekabio gained on news of a Google Cloud based infrastructure build-out and expectations that AI-discovered candidates will enter clinical trials.
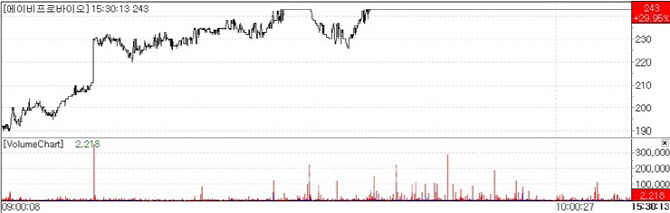 | | ABPROBIO share-price trend (Source: KG Zeroin MP Doctor) |
|
On Nov. 5, according to KG Zeroin’s MP Doctor(formerly Market Point), ABPROBIO closed at 243Won, up 29.95%(56Won) from 187 the previous session. The rally is attributed to progress with the multi-specific antibody anticancer drug being co-developed with Celltrion.
The two companies announced that they will present preclinical results for the multi-specific antibody drug CT-P72/ABP-102 at the Society for Immunotherapy of Cancer(SITC) 2025 meeting, held in Maryland, USA. The study was selected as one of the “Top 150” from over 1,300 submitted abstracts based on the originality of the preclinical research and its contribution to immunotherapy.
CT-P72/ABP-102 is a multi-specific antibody immuno-oncology therapy co-developed by Celltrion and U.S. company Abpro. It employs a T-cell engager mechanism that links HER2-expressing cancer cells with T cells to eliminate tumors. A T-cell engager is a bispecific antibody engineered to recognize tumor cells and T cells simultaneously, binding T cells directly to tumor cells to trigger an immune response and induce cancer-cell death.
In preclinical studies, CT-P72/ABP-102 demonstrated strong antitumor effects specifically in HER2-high tumors using a dual xenograft mouse model implanted with both high- and low-HER2 expressing cell lines. In addition, in non-human primate toxicology studies, no notable adverse effects were observed even at a high dose of 80 mg/kg, supporting an overall favorable safety profile.
Industry observers say CT-P72/ABP-102 has solid potential to become best-in-class within its modality, citing structural mitigation of toxicity-related adverse events and a superior therapeutic index(toxic dose vs. effective dose) versus peers. In animal studies comparing efficacy in Enhertu(trastuzumab deruxtecan)-resistant tumor models, CT-P72/ABP-102 showed significant tumor-growth inhibition, suggesting a potential new treatment option relative to existing therapies.
Miles D.(Seok), CEO and Chairman of Abpro, said, “The preclinical data developed in collaboration with Celltrion are highly encouraging. The strong antitumor activity in Enhertu-resistant models showcases the selectivity and engineering strengths of our DiversImmune platform.” He added that the team aims to complete an IND submission package with Celltrion this year and begin first-in-human testing next year.
Ildong Holdings: Limit-Up on GLP-1 Out-Licensing Hopes, “Commercialization to proceed in parallel with partnering” Ildong Holdings hit the upper price limit on expectations for technology transfer of its obesity therapy. The stock jumped 29.94%(2,710Won) from 9,050Won to 11,760Won, reclaiming the 10,000Won level about a month after falling from 10,300Won on Oct. 2.
The strength is linked to news that Ildong Pharmaceutical is conducting partnering meetings for ID110521156 at BIO-Europe 2025 in Vienna, Austria(through Nov. 5).
ID110521156 is an oral small-molecule GLP-1 receptor agonist(GLP-1 RA). Compared with injectable GLP-1 peptide drugs, it aims to differentiate on pharmacology, manufacturing efficiency and cost, and user convenience. The candidate maintains therapeutic plasma levels for over 18 hours with no systemic accumulation, enabling once-daily, long-term oral dosing?an attractive profile for commercialization.
In a recently completed Phase 1 study, the candidate achieved up to 13.8% weight loss over four weeks. No serious adverse events related to common GLP-1 side effects (gastrointestinal issues, hepatotoxicity) were observed. During BIO-Europe, Ildong plans to explore R&D collaboration and license-out opportunities with multiple global partners. Reflecting the L/O prospects, Ildong Pharmaceutical also rose 6.57%(1,740Won) to 28,400Won.
An Ildong Pharmaceutical official stated, “At recent global pharma-bio business events such as CPHI and BIO-Europe, we held partnering meetings on our pipelines including GLP-1 RA and P-CAB. We plan to pursue global commercialization, including potential license-out, in parallel with continued development of our candidates.”
Syntekabio, Global Infrastructure Build-Out + Clinical Entry Hopes for AI-Discovered Candidates Syntekabio, an AI-driven drug discovery company, jumped on the back of its Google Kubernetes Engine(GKE) based hybrid cloud build and expectations that AI-discovered candidates will advance into the clinic. The shares closed at 4,795Won, up 13.09%(555Won) from the prior session.
The company recently migrated its existing cloud environment to Google Cloud’s GKE, integrating proprietary AI drug-discovery platforms such as DeepMatcher® and its in-house AI Bio Supercomputing(ABS) Center with Google Cloud to optimize its AI capabilities. GKE, Google Cloud’s fully managed Kubernetes service, automates large-scale container workloads and offers a standardized environment for rapid, consistent deployment of AI applications?enabling Syntekabio to operate complex AI workloads with stability and scalability.
Separately, Syntekabio and Australia’s QIMR Berghofer Medical Research Institute(QIMRB) recently jointly identified a final candidate for an AI-based COPD therapy, and are preparing a patent filing as a step toward commercialization. Once the patent is filed, the company expects to initiate clinical development?its first clinical program for a candidate discovered via its AI platform.
A Syntekabio representative said, “Today’s share-price strength reflects our transition to Google Cloud for AI drug discovery and expectations for clinical entry of our internally discovered candidates. We are also conducting a shareholder allotment rights offering to fund platform upgrades, business expansion, and debt repayment. Successful completion should strengthen our balance sheet and secure growth drivers going forward.”










