[Seok Ji-hoen, Edaily Reporter] Biosolution, a developer of cell therapies for osteoarthritis, announced on March 12 that it participated in the American Academy of Orthopaedic Surgeons (AAOS 2025) annual meeting, held in San Diego from March 10~14th. The company presented key clinical results from its next-generation knee cartilage regeneration cell therapy, CartiLife, including final data from its Phase 3 clinical trial in South Korea and interim results from its Phase 2 trial in the United States.
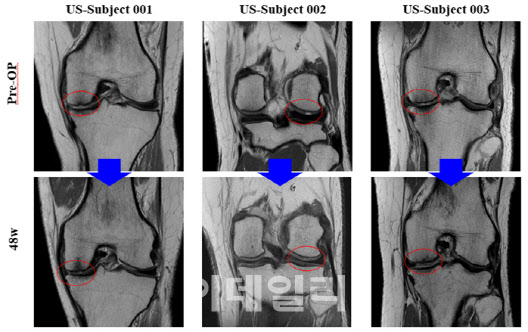 | | MRI scans of U.S. patients before and after the procedure showed that cartilage defects were filled by the 48-week mark. (Photo provided by Bio Solution.) |
|
Founded in 1933, the AAOS is the world‘s largest orthopedic association, with more than 40,000 members. Last year’s event attracted over 600 global companies, making it a key annual conference for both academia and industry. At the conference, Bio Solution introduced major clinical data on CartiLife, including the 48-week primary efficacy results from the South Korean Phase 3 trial and the 96-week long-term follow-up results. The company also shared preliminary findings from its ongoing data analysis of the U.S. Phase 2 trial.
Regarding the South Korean Phase 3 trial, Bio Solution highlighted MRI images taken at 24, 48, and 96 weeks, demonstrating CartiLife’s consistent efficacy compared to microfracture surgery. The P-value, which measures statistical significance, was recorded at 0.0131, well below the commonly accepted threshold of 0.05. Additionally, KOOS scores, which assess pain and mobility, showed continuous improvement over 24, 48, and 96 weeks. The cartilage regeneration effect was observed not only in younger patients but also in individuals over 50 and those with osteoarthritis.
For the U.S. Phase 2 trial, Bio Solution released MRI images from select patients, visually confirming cartilage regeneration in defect areas up to 48 weeks post-procedure. Key evaluation metrics, including KOOS (pain and mobility improvement), VAS, and IKDC scores, also demonstrated consistent improvement over 24 and 48 weeks.
Previously, Bio Solution reported that the MOCART score at 48 and 96 weeks post-surgery in the South Korean Phase 3 trial showed statistically significant improvement compared to the active control group (microfracture surgery). KOOS total score changes at these time points also confirmed non-inferiority to the control group. Secondary efficacy indicators, such as IKDC score (assessing knee function and activity) and KOOS score (measuring pain and daily activity performance), showed statistically significant differences, with safety also confirmed.
“This conference allowed us to highlight CartiLife’s superior cartilage regeneration capabilities and expand our network with interested pharmaceutical companies,” said Bio Solution CEO Lee Jung-sun. “Following the AAOS, we have continued meetings to strengthen partnerships. We will do our utmost to ensure that this and future global conferences lead to successful license-out agreements for CartiLife.”
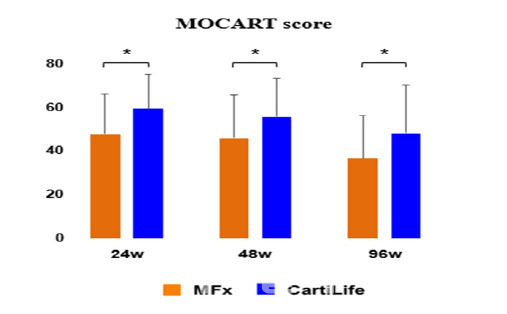 | | MRI analysis data from the South Korean Phase 3 trial confirmed sustained cartilage integrity compared to microfracture surgery. (Data provided by Bio Solution.) |
|




![2% Royalty Shock at Alteogen Ripples Through Korean Biotech[K-Bio Pulse]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/01/PS26012200181b.jpg)



!['2% 로열티'가 무너뜨린 신뢰…알테오젠發 바이오株 동반 하락[바이오맥짚기]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/01/PS26012201091b.jpg)


