Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[Lim Jeong-yeo, Edaily Reporter] On the 19th, shares of Pharos iBio, Oncocross and MedPacto were strong in the Korean biopharma sector. Although these names had been sidelined because of weak earnings, news that they have been steadily strengthening their fundamentals helped turn investor sentiment.
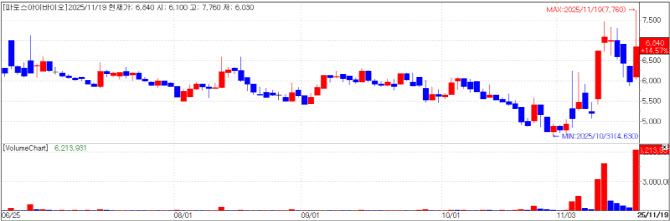 | | Pharos iBio chart(Cred=KG Zeroin MP Doctor) |
|
Pharos iBio: license-out expectations AI-driven drug discovery company Pharos iBio closed at 6,840 won, on Wednesday, up 14.57% (870 won) from the previous session. The move is seen as reflecting expectations for license-out deals after PharmEdaily‘s premium pay-to-read article titled
was published.
Among the company’s pipeline of new drug candidates, Pharos iBio has said it has registered PHI-101, for which it has obtained phase 1 clinical data, with the World Health Organization under the nonproprietary name “lasmotinib” and has begun to pursue license-out opportunities. In addition, PHI-501 has received approval of its phase 1 clinical trial plan (IND), with dosing scheduled to begin this month.
Through its “Chemiverse” platform, Pharos iBio identifies potential candidate compounds and then uses its own synthesis lab and wet lab to confirm whether those compounds actually work. Substances synthesized in-house are first validated through cell-based experiments, after which preclinical development is outsourced to external contract research organizations (CROs).
“Rather than relying on a single pipeline, we are steadily preparing three compounds—PHI-101 (lasmotinib), PHI-501 and PHI-201,” said Pharos iBio Chief Financial Officer Moon Sung-won. “For our last pipeline, PHI-201, we are in the lead optimization stage to derive the final candidate, and we plan to seek a license-out deal after preclinical testing.”
He added, “This approach takes into account the financial burden of running three clinical programs at the same time. In the case of PHI-201, we are conducting research having already identified a potential acquirer.”
At Pharos iBio, CEO Yoon Jung-hyuk leads platform R&D, while co-founder and President Nam Ki-yeob, as head of new drug development, oversees preclinical and clinical development.
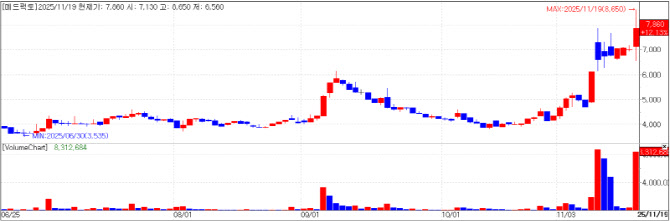 | | MedPacto chart(Cred=KG Zeroin MP Doctor) |
|
MedPacto: immuno-oncology candidate ‘MP010’ Immuno-oncology developer MedPacto, Inc. (MedPacto) closed at 7,860 won, on Wednesday, up 12.13% (850 won) from the previous day. The company announced that its new immuno-oncology candidate “MP010” has been selected as a new project to receive funding under the second round of the 2025 National New Drug Development Program, overseen by the Korea Drug Development Fund (KDDF).
MP010 selectively binds to “EDB-FN,” which is overexpressed in the tumor microenvironment (TME), while also targeting “TGF-β,” a key regulator of the TME that drives immune escape and metastasis in cancer. It is described as a first-in-class innovative drug candidate.
In preclinical studies, MedPacto has confirmed that MP010 monotherapy yields high complete response (CR) rates, long-term survival and immune memory effects in difficult-to-treat tumors such as pancreatic cancer and triple-negative breast cancer. With this project selection, the company plans to move forward with obtaining investigational new drug (IND) clearance for MP010 and advancing its early clinical development.
“Since we need to generate 3 billion won in revenue this year to maintain our listing status, we are steadily posting sales through services such as distribution of pharmaceuticals and health functional foods, as well as genomics analysis,” said MedPacto Head of Management Division Jang Min-hu. “Expectations are also building because ‘vactosertib,’ our long-standing core pipeline asset, recently delivered positive phase 1 osteosarcoma data.”
“Our current goal is to leverage the osteosarcoma clinical data for vactosertib and secure an early license-out deal for MP010, which is slated to enter clinical trials next year,” he added. “To that end, we attended BIO-Europe and the Society for Immunotherapy of Cancer (SITC) meeting held in Vienna, Austria, earlier this month and are now in talks with companies we met on-site.”
It is to be noted that Theragen Etex, MedPacto‘s largest shareholder, is pursuing to sell its stake in the company. The indicative sale price disclosed in November last year was 35.7 billion won, though it could be affected by share price movements.
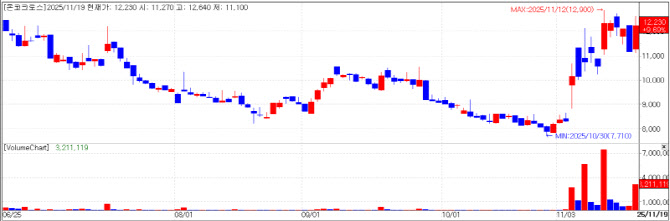 | | Oncocross chart(Cred=KG Zeroin MP Doctor) |
|
Oncocross: expanding global partnerships for protein analysis service AI-driven drug discovery company Oncocross closed at 12,230 won, on Wednesday, up 9.69% (1,080 won) from the previous session. The stock appears to have drawn attention after CEO Kim Yi-rang took part in a biotech roundtable hosted by Paju City, where he sat alongside speakers from the Broad Institute in the United States, Japan’s National Cancer Center and Australia‘s Children’s Medical Research Institute.
Once focused mainly on drug repurposing services through its “Raptor AI” platform, Oncocross is partly shifting its business mix. Recently, it has been highlighting “OncoFind AI,” a liquid biopsy platform that detects cancer via blood analysis. Development is under way, and the company expects to begin commercializing the platform from next year.
Oncocross has also recently launched a protein analysis service, securing a new revenue stream. The aim is not only to generate sales, but also to build a larger protein dataset to further advance its AI capabilities.
“To secure large numbers of protein samples, we are signing MOUs with hospitals and research institutes, and we plan to continue discussions with the Children‘s Medical Research Institute in Australia as well,” CEO Kim said.





![2% Royalty Shock at Alteogen Ripples Through Korean Biotech[K-Bio Pulse]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/01/PS26012200181b.jpg)



!['2% 로열티'가 무너뜨린 신뢰…알테오젠發 바이오株 동반 하락[바이오맥짚기]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/01/PS26012201091b.jpg)


