Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[Kim Jiwan, Edaily Reporter] On the 1st, Korea’s biotech and pharmaceutical sector drew strong investor attention as companies with commercialization-driven revenue in sight took center stage.
 | | Kim Soo-yoon, head of the Plant Cell Culture Team, is mass-culturing plant cells. (Courtesy of Bio FD&C) |
|
Bio FD&C boosted expectations for commercialization and export expansion as its plant cell based next-generation regenerative ingredient PDRN entered the global cosmetics OEM/ODM market in earnest through Kolmar Korea.
Curosell is in the spotlight as its “Anbalcel,” Korea’s first CAR-T therapy, awaits regulatory approval with the possibility of reimbursement, with a decision expected within the year.
ST Pharm is poised for earnings momentum as Novartis’s cholesterol-lowering drug “Leqvio” received U.S. FDA approval for monotherapy, expanding its patient base. ST Pharm has been supplying the drug’s core oligonucleotide ingredient since early clinical trials and will continue as a long-term supplier.
All three companies, leveraging strong technology and global networks, are expected to achieve rapid commercialization and revenue growth, drawing concentrated interest from investors.
Bio FD&C PDRN Set to Disrupt Cosmetics OEM/ODM Market Bio FD&C garnered market attention on news that its plant cell based polydeoxyribonucleotide (PDRN) will enter the domestic and global cosmetics OEM/ODM market via Kolmar Korea.
 | | Plant cells are being cultured in a bioreactor. (Courtesy of BioFD&C) |
|
The company’s share price rose 3.20% (610 won) to close at 19,690 won. Bio FD&C began supplying major domestic cosmetics companies in May and will begin U.S. exports in the second half. From next year, PDRN is expected to be incorporated into multiple global cosmetics brands, led by Kolmar Korea.
PDRN, DNA fragments with skin regeneration and anti-aging effects, is traditionally extracted from animals such as salmon or trout, but Bio FD&C possesses rare technology to extract it from plant cells. The company operates with eight plant cell lines, improving yield and standardizing mass production, and has also developed a rose cell based PDRN prototype.
Kolmar Korea, the No. 1 cosmetics OEM/ODM firm in Korea, works with numerous domestic and global brands. Bio FD&C’s raw material supply is expected to accelerate large-scale commercialization and entry into global distribution channels.
CEO Sang-Hyun Mo stated, “This collaboration with Kolmar Korea is not just supply?it’s a signal for global market expansion. Plant-derived PDRN offers safety and cost competitiveness as a next-generation regenerative ingredient.”
Curocell’s ‘Anbalcel’ Nears Approval as Korea’s First CAR-T Curocell’s “Anbalcel” (CRC01), Korea’s first CAR-T therapy, is nearing approval, lifting the company’s share price by 2.78% (1000 won) to 37,000 won.
Curocell filed for product approval in December last year with Korea’s Ministry of Food and Drug Safety (MFDS) for relapsed or refractory diffuse large B-cell lymphoma (DLBCL). At the time of submission, the drug’s name was “Limcatoju.”
Industry expectations are that approval will be decided within the year. Approval would make it the first domestically developed CAR-T therapy. Currently, Novartis’s “Kymriah” is the only CAR-T therapy approved for this indication in Korea.
Anbalcel is expected to commercialize rapidly after approval. An industry source said, “Limcatoju (Anbalcel) is part of the pilot program for simultaneous new drug approval, reimbursement, and price negotiations, which will allow for immediate insurance coverage and greatly improve patient access.”
A Curocell representative added, “If approved, Curocell will strengthen its position as Korea’s first commercial CAR-T therapy developer.”
ST Pharm Benefits from Leqvio’ Monotherapy ST Pharm continued its upward momentum, closing 2.80% (2,500 won) higher at 91,800 won, as it emerged a prime beneficiary of the U.S. FDA’s approval of Novartis’s “Leqvio” (inclisiran) for monotherapy in cholesterol management.
 | | A view of ST Pharm’s Banwol facility (Photo courtesy of ST Pharm) |
|
This approval allows Leqvio to be prescribed to a broader patient population, boosting ST Pharm’s outlook as the supplier of its core oligonucleotide ingredient.
First approved by the FDA in 2021, Leqvio generated $754 million (1 trillion won) in sales last year, achieving blockbuster status. It is now approved in over 80 countries, including Korea.
ST Pharm has supplied oligonucleotides since early clinical development and expanded commercial production capacity after European and U.S. approvals. The recent label expansion is expected to directly drive ST Pharm’s sales growth.
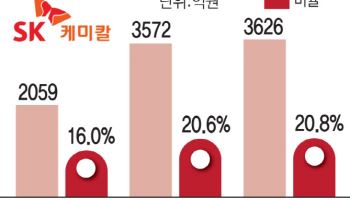
Leqvio-related revenue for ST Pharm reached 60 billion won in 2023, 83 billion won last year, and is projected to surpass 100 billion won in 2024. Completion of the second oligo-dong facility has expanded annual production to 14 mol (2.3~7 tons), with order backlog reaching 440 billion won in July up more than 200 billion won from year-end 2023.
An industry insider noted, “The global demand growth and label expansion for Leqvio will have long-term positive effects on ST Pharm.”






![Selvashealthcare Hits Upper Limit on News of Business Talks With Wall Street Firm[K-Bio Pulse]](https://image.edaily.co.kr/images/vision/files/NP/S/2025/12/PS25121100282b.jpg)

![이혁준 서울대병원 교수 "암환자 식단관리앱 힐리어리 예후 바꿀 첫 도구 "[전문가 인사이트]](https://image.edaily.co.kr/images/vision/files/NP/S/2025/12/PS25121100299b.jpg)
!['상장 첫날 시총 2.8조' 에임드바이오, 코스닥 다크호스 급부상 [바이오맥짚기]](https://image.edaily.co.kr/images/vision/files/NP/S/2025/12/PS25120500172b.jpg)


