Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[이데일리 임정요 기자] On October 17, Asterasys gained the most traction in South Korean biopharma stock market after receiving a green light for its entry into the U.S. market. GC Cell also gained momentum on news of a domestic patent registration for its genetically modified immune cell therapy. Samsung Biologics saw a mild slide after its extraordinary shareholders’ meeting approved a spin-off plan.
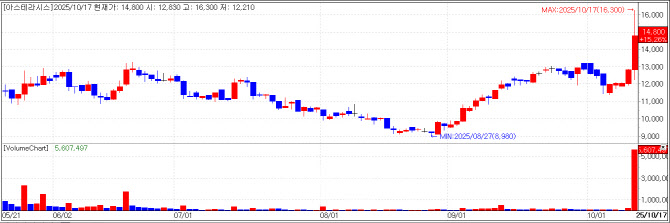 | | Asterasys chart(Cred=KG Zeroin MPDoctor) |
|
Asterasys to Begin U.S. Sales of “Coolfase” Next Year According to KG Zeroin MPDoctor (formerly MarketPoint), Asterasys closed 15.26% higher at 14,800 won, up 1,960 won from the previous day, following news that its flagship product Coolfase obtained U.S. FDA 510(k) clearance.
Coolfase is a monopolar radiofrequency (RF) aesthetic medical device equipped with proprietary Dynamic Cooling Control (DCC) technology that reduces pain during lifting procedures. The U.S. clearance is expected to significantly boost Asterasys’ revenue growth.
As confirmed by Edaily, most of Asterasys’ revenue through last year came from its high-intensity focused ultrasound (HIFU) device Liftera and related consumable cartridges. Liftera accounted for 19.9 billion won (69%) of the company’s 28.8 billion won total sales in 2023.
In contrast, RF devices --the bipolar Futera and monopolar Coolfase -- together generated 6.8 billion won, about 23% of total revenue. The upcoming U.S. launch is thus expected to increase the RF segment’s share of sales.
Coolfase received Korean MFDS approval as a medical device and launched domestically in April 2023. Since then, the company has secured regulatory approvals in Brazil (September 2023), Taiwan, Indonesia, and Thailand, accelerating its global expansion.
Asterasys said that it had met with several U.S. distributors at global aesthetic medical conferences such as IMCAS in Paris earlier this year. Multiple distributors reportedly expressed interest in handling U.S. sales of Coolfase, and Asterasys plans to sign with the most competitive partner and begin full-scale U.S. sales in 2026.
An Asterasys official stated, “Aside from us, only Classys has obtained FDA 510(k) clearance for a monopolar RF aesthetic device in the U.S.,” adding, “This marks the beginning of full-fledged competition in the world’s largest market.”
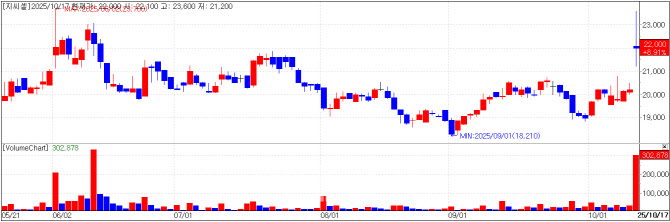 | | GC Cell chart(Cred=KG Zeroin MP Doctor) |
|
GC Cell Registers Domestic Patent for CAR-NK ‘GCC2005’ GC Cell closed 8.91% higher at 22,000 won, up 1,800 won, after announcing domestic patent registration for its chimeric antigen receptor (CAR)-modified natural killer (NK) immune cells.
The patented technology aims to treat CD5-positive tumors, a type of cancer cell, more effectively. It enhances immune cell activity to maximize anticancer efficacy and allows the cells to survive and proliferate longer in the body, offering new therapeutic potential for patients with CD5-positive malignancies such as lymphocytic leukemia.
GC Cell said this patent represents a core technology for its CAR-NK therapy candidate GCC2005, based on a differentiated platform with enhanced cell viability and proliferation. The therapy is currently in a Phase 1 trial in Korea for patients with relapsed or refractory NK and T-cell malignancies.
According to the company, competing CAR-T therapies for similar indications face limitations such as proliferation restriction due to intercellular attack, risk of tumor cell mixture, and damage to normal T cells. In contrast, GCC2005 -- derived from healthy donor NK cells -- is expected to overcome these challenges and deliver superior tumor-killing ability.
CEO Sung-Yong Won commented, “This patent registration is a meaningful milestone that officially recognizes GC Cell’s immune cell therapy capabilities,” adding, “We will continue to strengthen our global competitiveness through innovative cell therapy R&D.”
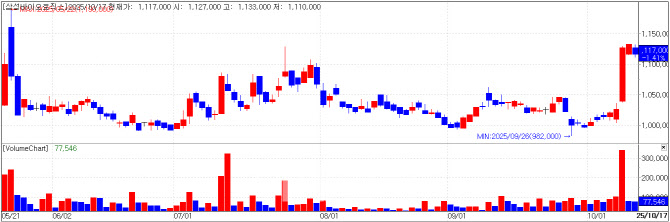 | | Samsung Biologics chart(Cred=KG Zeroin MP Doctor) |
|
Samsung Biologics Shareholders Approve Spin-Off Samsung Biologics closed at 1,117,000 won, down 1.41% (16,000 won) from the previous day on October 17, as the company’s extraordinary shareholders’ meeting approved its spin-off plan. This followed a 9.7% surge (100,000 won) on October 15.
The effective date of the spin-off is November 1, with trading suspended from October 30 to November 21. The surviving and new entities are scheduled for relisting on the KOSPI on November 24.
This spin-off is anticipated to fully separate the company’s biopharmaceutical contract development and manufacturing (CDMO) business from its biosimilar and new drug investment division. The surviving entity, Samsung Biologics, will become a “pure-play” CDMO focusing on contract manufacturing for clients.
The new holding company, Samsung Epis Holdings, will inherit 100% ownership of Samsung Bioepis, managing subsidiaries and pursuing new investments.
The share distribution ratio is 0.6503913 (Samsung Biologics) to 0.3496087 (Samsung Epis Holdings), based on net asset book values as of the end of Q1 2025.
CEO John Rim stated, “This spin-off will allow the CDMO and biosimilar businesses to be independently listed and transparently valued in the capital market. Each company will strengthen its core expertise and competitiveness to enhance shareholder value.”





![Xcell Therapeutics Hits Upper Limit...Celemics·QuadMedicine ↑[K-Bio Pulse]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/02/PS26020600366b.jpg)






