Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[Song Young Doo, Edaily Reporter] Shares of QuantaMatrix surged after the company was selected as a recipient of a U.S. government-led infectious disease response fund. This marks the first time a Korean company has been directly awarded research funding from the program. Meanwhile, Proteina, which debuted on KOSDAQ last month, rallied sharply despite a lock-up expiration as foreign investors, including a major U.S. fund, increased their stakes. D&D Pharmatech also advanced as the U.S. Food and Drug Administration(FDA) signaled it may ease clinical trial standards for MASH(metabolic dysfunction-associated steatohepatitis) therapies.
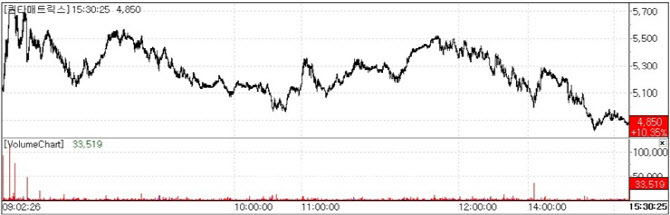 | | QuantaMatrix Stock Trend.(Source=KG Zeroin MP Doctor) |
|
QuantaMatrix Selected for U.S. CARB-X Funding On August 29, QuantaMatrix closed at 4,850 won, up 10.35%(455 won) from the previous session, according to KG Zeroin’s MP Doctor (formerly MarketPoint). The jump was driven by news that QuantaMatrix had been chosen for a U.S. government-backed initiative.
Earlier that day, PharmEdaily, E-Daily’s premium pharma and biotech news service, broke the story:
“[Exclusive] QuantaMatrix Becomes First Korean Firm to Secure $20B U.S. Infectious Disease Grant.”QuantaMatrix, a clinical microbiology diagnostics company, was selected for funding by CARB-X, an international public-private partnership led by BARDA under the U.S. Department of Health and Human Services. The project, valued at up to $13.9 million over four years, will provide up to $5.7 million for technology development and up to $8.2 million for commercialization preparation, pending milestone reviews.
Since its launch in 2017, CARB-X has funded over 100 research projects, some of which have advanced to commercial products. Investors include the U.K. Department of Health, Health Canada, the Bill & Melinda Gates Foundation, and the Wellcome Trust. The U.S. government invests at least $1 billion annually to combat antimicrobial resistance, with sepsis accounting for about 20% of global deaths.
QuantaMatrix will use the grant to develop a technology capable of detecting more than 50 pathogens from just 1 milliliter of neonatal blood within six hours a challenge long considered nearly impossible due to sample limitations and technical hurdles. Its proprietary QMAP platform, which analyzes multiple diagnostic markers simultaneously using coded microbeads, was a key factor in its successful bid against competition exceeding 20-to-1.
CEO Kwon Sung-hoon noted, “Extracting 1 milliliter of blood from a newborn amounts to just 20 drops. Detecting more than 50 bacterial species under such conditions seemed almost impossible. But last year, our Nature paper on uRAST demonstrated the feasibility, and that was highly valued in the selection process.”
Proteina Rallies on Global Fund Buying Proteina posted the sharpest gain among biotech stocks that day, closing at 17,500 won, up 14.38%(2,200 won) from 15,300 won. The surge came even as 1.6 million shares were released from lock-up. According to the company, most of the shares were absorbed by foreign institutions, including what it described as “the world’s largest U.S. fund.”
A company official said, “Although the stock dipped briefly in the morning as lock-up shares hit the market, foreign ownership rose by more than 5% during the day, and major global institutions increased their holdings, driving the sharp rally.”
Proteina, a protein-protein interaction(PPI) big data firm, develops biomarker-based diagnostics using its PPI platform. The company has worked on a companion diagnostic(CDx) to identify patients for AbbVie’s blood cancer therapy venetoclax, which it presented at last year’s American Society of Hematology meeting. Proteina also plans to acquire a U.S. CLIA lab this year and launch diagnostic services in the U.S. by the second half of 2026.
D&D Pharmatech Strengthens on FDA MASH Trial Update Shares of D&D Pharmatech also climbed, rising 11.15%(15,800 won) to 157,500 won, marking six consecutive sessions of gains and approaching its 52-week high of 159,200 won.
The rally followed reports that the FDA may accept non-invasive tests as clinical endpoints in MASH drug trials, easing reliance on invasive liver biopsies, which carry risks of pain, bleeding, and sample distortion. Analysts expect the shift to shorten development timelines and improve success rates.
D&D Pharmatech is developing DD01, a once-weekly subcutaneous injection currently in Phase 2 trials. The company’s candidate has shown a 62.3% reduction in liver fat, outperforming Eli Lilly’s drug(57%) and matching the performance of MSD’s pipeline candidate.



![Xcell Therapeutics Hits Upper Limit...Celemics·QuadMedicine ↑[K-Bio Pulse]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/02/PS26020600366b.jpg)






