Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[Yu Jin-hee, Edaily Reporter] SEOUL, South Korea-Shares of Daehwa Pharmaceutical surged to their highest point this year, driven by optimism around sales growth in China. Cytogen and NIBEC also posted significant gains on April 14, reflecting investor confidence in anticipated business developments.
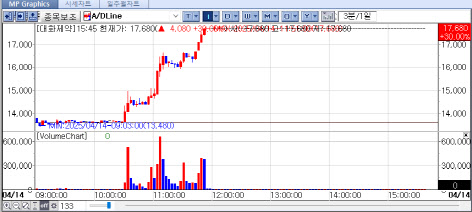 | | Daehwa Pharmaceutical‘s Recent Stock Price Flow. [Source=KG Zeroin’s MP Doctor] |
|
Daehwa Pharma hits year-high on positive PharmEdaily news Daehwa Pharma‘s shares climbed the daily limit of 30%, closing at 17,680 won, up from the year’s opening price of 10,900 won-a 62.2% increase despite broader market challenges driven by negative global sentiments, particularly from the U.S.
The stock surge was triggered by a pay-to-read PharmEdaily article published March 10, which highlighted Daehwa Pharma‘s launch of Liporaxel in China, described as “the world’s first oral cancer drug.” After the article became freely accessible online, subsequent similar reports further propelled investor interest, significantly boosting the stock price.
Liporaxel, a novel oral formulation of the blockbuster cancer drug Taxol (paclitaxel), is marketed in China by Haihe Biopharma and local distributor 3S Bio. Haihe Biopharma received approval from China‘s National Medical Products Administration (NMPA) for Liporaxel as a treatment for gastric cancer last September and began sales in mid-February.
Unlike intravenous formulations, Liporaxel requires no solvents, injection equipment, or premedication typically associated with infusion therapies, enhancing patient convenience significantly. The drug incorporates Daehwa’s proprietary lipid-based self-emulsifying drug delivery platform (DHLASED).
Daehwa Pharma anticipates Liporaxel will significantly boost annual sales, already included in last year’s revenue of 141.4 billion won. The company has received a $25 million licensing fee from its Chinese partner, Haihe, of which $13 million has been paid so far. Daehwa is set to receive royalties for 10 years post-launch and plans to expand Liporaxel‘s indications from gastric cancer to metastatic and recurrent breast cancer.
“We are actively exploring ways to enhance sales, such as securing local insurance coverage in China,” a Daehwa Pharma official said. “Considering the success of Lipusu, a lipid-based intravenous paclitaxel formulation by Lvye Pharma, we expect significant growth for Liporaxel.”
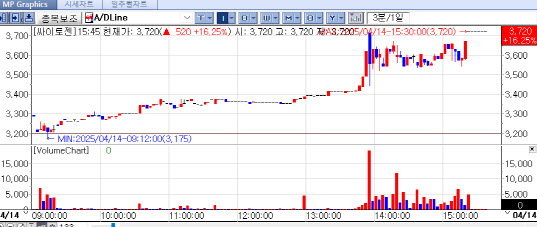 | | Cytogen‘s Recent Stock Price Flow. [Source=KG Zeroin’s MP Doctor] |
|
Cytogen and NIBEC shares also rise on positive expectations Cytogen shares rose 16.25% to 3,720 won, reflecting optimism around improving earnings. The company expects its U.S.-based CLIA-certified laboratory, ExperTox, to benefit significantly from a recent court decision invalidating the FDA’s Laboratory Developed Test (LDT) regulations.
Cytogen specializes in liquid biopsy technology, diagnosing cancer and other diseases through non-invasive methods like blood or urine tests. ExperTox, acquired by Cytogen in 2022, provides LDT approval support and joint development services to Korean firms seeking to enter the U.S. market. With the regulatory constraints lifted for at least one year, Cytogen expects heightened demand for these services.
NIBEC also gained 13.67%, closing at 21,200 won, driven by investor anticipation of its first global licensing deal for peptide-based drug candidate NP-201. NP-201 targets fibrosis through a unique mechanism promoting tissue regeneration, potentially applicable to diseases like idiopathic pulmonary fibrosis and renal fibrosis.
“NIBEC has received significant interest from international firms for NP-201 due to its therapeutic potential,” said Han Yang Securities analyst Oh Byeong-yong. “Discussions with a U.S. pharmaceutical company have progressed significantly, and a formal licensing agreement is likely to be finalized in the first half of this year.”




![Xcell Therapeutics Hits Upper Limit...Celemics·QuadMedicine ↑[K-Bio Pulse]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/02/PS26020600366b.jpg)






