Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[Lim Jeongyo, Edaily Reporter] OliPass and NatureCell stocks closed at the upper limit in the Korean biopharma and healthcare sector, Tuesday. OliPass, despite facing a possible designation as an “under watch” stock due to subpar financial performances, surged to the daily cap, drawing inquiry from the Korea Exchange. Meanwhile, NatureCell soared for a second straight session after announcing its knee osteoarthritis treatment JointStem was designated as a Breakthrough Therapy by the U.S. Food and Drug Administration(FDA). Also rising were Onconic Therapeutics and its parent company Jeil Pharmaceutical, on back of Onconic’s imminent receival of milestone payment from Chinese Livzon Pharmaceutical Group.
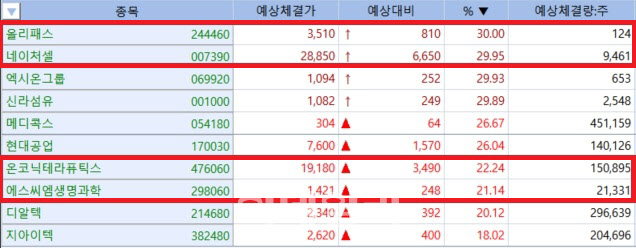 | | Kosdaq high-performers on March 25(Source: KG Zeroin MP Doctor) |
|
OliPass slapped with trading inquiry amid ownership changesAccording to KG Zeroin’s MP Doctor (formerly MarketPoint), OliPass ended the day up 810 won, or 30%, at 3510 won. While about 40,000 shares were on the buy side, just 124 shares actually changed hands due to a lack of willing sellers.
The rally seemingly had no apparent trigger, with OliPass potentially marked as a financial underperformer requiring further investment prudence. The unexpected price surge prompted the Korea Exchange’s KOSDAQ division to request a clarification disclosure by 6 p.m., Wednesday.
OliPass is a preclinical biotech firm developing non-narcotic analgesics based on RNA therapeutics. Listed in 2019, the company’s research and development progress remains in Phase 2a. OliPass has nabbed two license-out deals, one of which was prematurely terminated.
The company posted just 1.9 billion won in sales last year, falling short of the 3 billion won requirement to maintain KOSDAQ listing status. OliPass delayed submission of its audit and annual business report from the original March 21 to April 7.
The auditing firm KPMG Samjong said it was unable to conclude on OliPass’ status as a going concern or the validity of its intangible asset valuations.
OliPass is also undergoing significant changes to its shareholders and management layout. The company’s principal shareholder changed from former CEO Jeong Shin to an investment body called Invest Partners No. 1 in June 2024, following the latter’s cash injection of 3 billion won to OliPass. This was after a 6-month arduous ammendments and reductions regarding the volume of the investment.
Only six months later in Jan. 2025, OliPass announced another capital raise of 4 billion won, this time with Genocure. The targeted raise amount was scaled down from an initially planned 20 billion won. Genocure will step up as the new principal shareholder this Friday, upon completion of payment.
Genocure CEO Oh Bo-kyung and Vice Chairman Kim Tae-hyun have preemptively joined OliPass’s board as inside directors as of Nov. 2024. Earlier this year, Genocure and OliPass entered an exclusive domestic distribution partnership for PDRN pharmaceuticals.
Since Dec. 2024, OliPass is run by co-CEOs Lee Jin-han and Son Hyung-seok. Lee is the CEO of cosmetics brand WhenBeauty, and is currently OliPass’s board chair. Son, a representative tax accountant at DaHyun Tax, previously acquired 2 billion won worth of OliPass convertible bonds(CB) in Mar. 2023.
Edaily was unable to receive a response from OliPass regarding its future management plans.
NatureCell hits ceiling for second consecutive dayNatureCell, a stem cell therapy developer, closed up 6,650 won, or 29.95%, at 28,850 won on Tuesday, marking the second consecutive day of hitting trading ceiling in South Korean bourse. The rally was triggered by the company’s degenerative knee arthritis treatment JointStem receiving Breakthrough Therapy Designation (BTD) from the U.S. FDA, according to NatureCell.
JointStem is an autologous stem cell therapy derived from a patient’s own adipose tissue. It positions itself as a “fundamental cure” that regenerates knee joints with a single injection without surgery. JointStem has completed Phase 3 trial in Korea in 2020. Ongoing U.S. trials are currently in Phase 2b/3a. R, Bio is responsible for domestic R&D and approvals, while NatureCell oversees the U.S. project.
JointStem was rejected by the Ministry of Food and Drug Safety(MFDS) in 2023, but R, Bio reapplied in 2024 and is awaiting results. The U.S. approval prospects may likely reshape domestic sentiment towards JointStem.
In preparation for its U.S. application, NatureCell appointed former FDA official Dr. Jung Sang-mok as the global clinical development and regulatory affairs executive. Dr. Jung holds a Pharm.D. from the University of Illinois at Chicago and spent 21 years at the FDA as senior researcher and more.
To this date, stem cell treatments approved by the FDA is known to be limited to hematopoietic stem cell transplants. Domestically, there are four approved stem cell therapies: Pharmicell’s HeartiCellgram (myocardial infarction, 2011), Medipost’s Cartistem (osteoarthritis, 2012), Anterogen’s Cupistem (Crohn’s-related fistulas, 2012), and CorestemChemon’s Neuronata-R (ALS, 2014). No new stem cell therapies have been approved in Korea since 2014.
Jeil Pharmaceutical and Onconic rise on royalty prospectJeil Pharmaceutical’s innovative drug development subsidiary Onconic Therapeutics disclosed that it has invoiced its Chinese partner for around 2.2 billion won in license fees, pushing its stock up sharply. Onconic announced on Monday that it had completed the transfer of manufacturing technology for Zastaprazan (Zacube tablets) to Livzon Pharmaceutical Group, per their license-out agreement in 2023.
On Tuesday, the day after the announcement, Onconic closed up 3,490 won (22.24%) at 19,180 won. Jeil Pharmaceutical, which holds a 46.28% stake in Onconic, also gained 990 won (8.68%) to close at 12,390 won.
Zastaprazan is a P-CAB drug (potassium-competitive acid blocker) used to treat GERD (gastroesophageal reflux disease). It is the third player to join the market after HK inno.N’s Tegoprazan (K-CAB) and Daewoong’s Fexuprazan (Fexuclu).
In 2023, Onconic generated 9 billion won in licensing revenue and 5.8 billion won in domestic product sales. Onconic’s sales are anticipated to grow further once the company makes global expansion. For Jeil Pharmaceutical, Onconic is a key growth engine for the future.
Separately, Jeil held its general shareholders’ meeting on Tuesday. appointing Han Sang-chul, the eldest son of the company’s founding family, as co-CEO alongside incumbent Sung Seok-je. His younger brother, Han Sang-woo, was named an internal director. Both now sit on the board.
“We aim to strengthen management accountability to enhance corporate competitiveness,” a company spokesperson said.







![에이프릴바이오, 임상 결과 전 내부자 매도…셀비온·뉴로핏은 강세[바이오 맥짚기]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/02/PS26021100299b.jpg)


