Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[[Yu Jin-hee, Edaily Reporter] SEOUL, South Korea-On the 11th, Korea’s stock market saw significant gains from Daehwa Pharmaceutical and Daebong LS, underscoring the resilience of pharmaceutical and biotech firms. These two companies reaffirmed that markets never ignore solid growth catalysts, as evidenced by Daehwa’s China export momentum and Daebong LS’s advancements in obesity treatments.
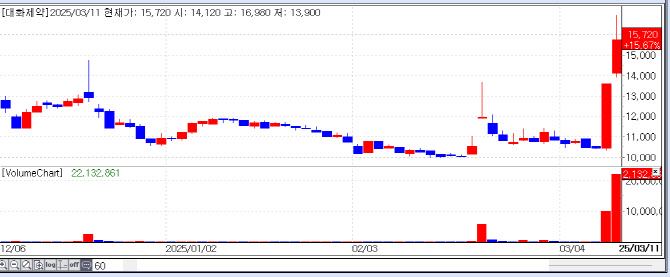 | | Daehwa Pharmaceutical‘s Recent Stock Price Flow. [Source=KG Zeroin’s MP Doctor] |
|
Data from financial analytics firm KG Zeroin MP Doctor showed Daehwa Pharmaceutical stock jumped 15.67%, closing at KRW 15,720. Daebong LS and Huons Global also posted strong gains, rising 29.96% and 15.26%, respectively.
Daehwa Pharmaceutical’s rally followed the release of a PharmEdaily report highlighting the official launch of Liporaxel, the world‘s first oral form of paclitaxel, in China. The medication, developed with Daehwa’s patented DHLASED lipid-based drug delivery system, began sales in mid-February via Chinese partner Haihe Biopharma and local distributor 3S Bio, following regulatory approval by China‘s National Medical Products Administration (NMPA) in September 2024 for treating gastric cancer.
Liporaxel offers significant improvements over traditional intravenous cancer therapies, eliminating the need for solvents, intravenous equipment, and pre-treatment hospital stays. Daehwa Pharmaceutical expects Liporaxel to drive its annual revenue to KRW 200 billion, up from KRW 141.4 billion recorded in 2024, which included initial Liporaxel sales.
The company’s licensing agreement for Liporaxel in China totals approximately USD 25 million (KRW 36 billion), with USD 13 million (KRW 19 billion) already received. Daehwa will also earn milestone payments and royalties for ten years post-launch. The firm plans to extend Liporaxel’s indications beyond gastric cancer to metastatic and recurrent breast cancer, aiming to expand its market share.
“The Chinese market, the world’s second-largest for pharmaceuticals, represents a critical growth opportunity for us,” said a Daehwa spokesperson. “Broadening Liporaxel’s therapeutic scope will help sustain our sales momentum.”
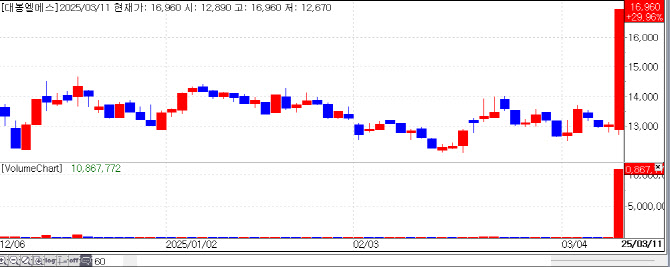 | | Daebong LS‘s Recent Stock Flow. [Source=KG Zeroin’s MP Doctor] |
|
Separately, Daebong LS gained attention after announcing a strategic collaboration with India‘s Shilpa Pharma Lifesciences Limited to manufacture the obesity treatment liraglutide. Daebong LS has developed technology to synthesize liraglutide, a GLP-1 receptor agonist recognized for effectively managing obesity and diabetes. This partnership is expected to improve efficiency, reduce costs, and enhance product quality.
Analysts anticipate significant opportunities in the global obesity treatment market, forecasted to reach KRW 100 trillion by 2030. They suggest that companies demonstrating differentiated products and robust R&D capacity will thrive, highlighting Huons Global’s subsidiaries, Huons and Huons Lab, as notable players in this promising sector.




![Hyundai Bio Hits Limit-Up, HLB Life Science Climbs on FDA NDA Plan[K-Bio Pulse]](https://image.edaily.co.kr/images/vision/files/NP/S/2025/07/PS25071300087b.jpg)

![더블유에스아이, ‘유봇’으로 의료로봇 도전장[인베스트 바이오]](https://image.edaily.co.kr/images/vision/files/NP/S/2025/07/PS25071300096b.jpg)
![인투셀, 특허이슈에 장외 하한가…퓨쳐켐, 진단제 국내 품목허가 추진[바이오 맥짚기]](https://image.edaily.co.kr/images/vision/files/NP/S/2025/07/PS25071000247b.jpg)
![[한주의 제약바이오]프로젠 "차별화된 비만약 연구 구두발표 선정"](https://image.edaily.co.kr/images/content/defaultimg.jpg)

