Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[Kim Saemi, Edaily Reporter] On the 26th, the bio and healthcare sector saw remarkable momentum: Bridge Biotherapeutics hit its upper limit for four consecutive trading days, while Abion and Telcon RF Pharmaceutical followed with three straight sessions of limit-up. Reyon Pharmaceutical, which had previously shown little price volatility, surprised the market with a 22% surge in a single day.
According to MP Doctor (formerly MarketPoint) by KG Zeroin, of the six stocks that hit the daily price ceiling on the KOSDAQ market that day, three were biotech firms: Abion (30%), Telcon RF Pharmaceutical (29.9%), and Bridge Biotherapeutics (29.78%). On the KOSPI, mid-sized drugmaker Reyon Pharmaceutical recorded the largest gain of the day, soaring 22.44%.
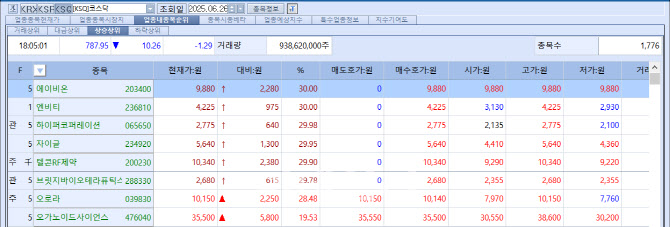 | | On the 26th, three out of six stocks that hit the daily price limit on the KOSDAQ market were biotech companies. (Source: KG Zeroin MP Doctor) |
|
Abion’s news of a $1.315 billion (approx. 1.8 trillion KRW) technology licensing deal for its antibody drug ‘ABN501’ drove both Abion and its largest shareholder Telcon RF Pharmaceutical to limit-up for three straight sessions. Bridge Biotherapeutics is also riding a streak of limit-ups after U.S. crypto-focused hedge fund Parataxis Holdings LLC acquired management control, quelling fears of delisting. Reyon’s rally came after news broke of a CDMO (contract development and manufacturing) deal for obesity treatments.
Bridge Bio: From Collapse to Comeback Bridge Biotherapeutics closed at 2,680 KRW on the 26th, up 615 KRW (29.78%) from the previous day. The company has now hit the daily upper limit for four consecutive sessions following the announcement of its management control transfer to U.S. digital asset investment firm Parataxis Holdings LLC. This marks a dramatic turnaround from earlier this year, when Bridge Bio suffered five consecutive sessions of limit-down after a failed Phase 2 trial of its idiopathic pulmonary fibrosis candidate BBT-877.
Analysts attribute the rally to investor optimism that the management change will lift the company from its status as a designated issue of concern and stave off the risk of delisting.
Bridge Bio was designated as such in March after failing to resolve persistent pre-tax losses from continuing operations. In April, BBT-877 failed to meet its primary endpoint in Phase 2, sending shares tumbling from 8,960 KRW to a low of 671 KRW-dropping it to penny stock territory.
Despite skepticism, the company successfully secured a strategic investor and raised 25 billion KRW. However, doubts remain over the synergy between the company’s original drug development pipeline and its new crypto-fintech direction. “It‘s unclear whether the coexistence of crypto and drug development will work out,” said one industry insider, “but it could definitely drive share price momentum.”
Investors are also closely watching whether Parataxis will follow through on its promised capital injection. Bridge Bio CEO Jeong-kyu Lee responded, “The capital payment will proceed without any setbacks.”
$1.8B Deal Lifts Abion, Telcon RF 3 Days Straight Abion opened limit-up on the 26th, and its top shareholder Telcon RF Pharmaceutical followed by hitting the ceiling at 9:11 a.m. The two companies have now posted three straight limit-up sessions following the June 24 announcement of Abion’s licensing deal for its antibody therapy ABN501, worth $1.315 billion (approx. 1.8 trillion KRW).
ABN501 targets claudin-3 (CLDN3), a protein overexpressed in solid tumors. The antibody drug candidate, which completed preclinical studies in April, selectively binds to CLDN3 at tight junctions between cancer cells, including in small cell lung cancer (SCLC). It is designed to be developed using various platforms such as antibody-drug conjugates (ADCs) and bispecific antibodies for a broad range of tumor types.
Though the licensee was not disclosed, industry sources suggest it is a U.S.-based ADC development company. In April, Abion had announced the signing of a term sheet for tech transfer with an unnamed American ADC firm.
The massive tech transfer is also expected to resolve concerns over Abion’s financial health. After exceeding the regulatory threshold for pre-tax loss versus equity capital in 2023, Abion was at risk of designation as a concern. Its ratio had climbed to 154% but was reduced to 58% by Q1 2024.
Abion had been preparing contingency plans such as converting outstanding convertible bonds (CBs) into common shares to bring the ratio below 50%. The upfront payment of $25 million (approx. 34 billion KRW) from this licensing deal now adds to the company’s financial stability.
“Abion has been undervalued for some time,” a company representative noted. “We believe there’s still upside potential ahead.”
Reyon Jumps 22% on CDMO Deal for Obesity Drug Reyon Pharmaceutical closed at 13,750 KRW, up 2,520 KRW (22.44%) from the previous session, after announcing a production contract for a modified GLP-1 receptor agonist (GLP-1RA) obesity and diabetes treatment. Such a large move is rare for the mid-cap firm, which posted annual sales in the 150 billion KRW range. The rally underscores surging investor interest in obesity-related therapeutics.
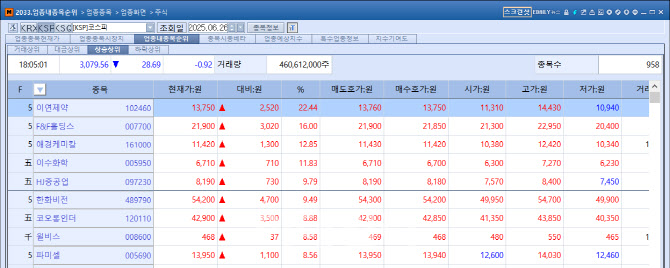 | | On the 26th, mid-sized pharmaceutical company Reyon Pharmaceutical surged 22.44% on the KOSPI, ranking first in daily gains. (Source: KG Zeroin MP Doctor) |
|
The company revealed it had signed a deal with SN Bioscience to manufacture finished GLP-1RA injectable products. SN Bioscience develops anticancer and obesity drugs using nanomedicine delivery technology.
Under the agreement, Reyon’s Chungju plant will handle the entire production process from drug substance manufacturing to fill-finish and final packaging. The facility will produce clinical trial samples through 2027 and will manufacture commercial batches post-approval.
Reyon had previously received GMP certification for finished drugs in August 2023, followed by CDMO-related GMP approval in November of the same year. While the company has signed multiple smaller-scale deals since, confidentiality clauses prevented public disclosure.
“We’ve had many orders that couldn’t be disclosed due to client or product confidentiality,” a Reyon official said. “This time, the visibility around both the client and the product likely drew more investor attention.” The official added, “We honestly didn’t expect this level of excitement around obesity drugs.It’s been quite overwhelming.”










![인도, 글로벌 저가 제네릭에서 바이오 허브로 탈바꿈[제약·바이오 해외토픽]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/02/PS26020700379b.jpg)