Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[Seok, Ji-hoen, Edaily Reporter] On Sept. 11, South Korea’s biotech stock market showed mixed performances. Curacle hit the daily upper limit after announcing a pipeline development partnership with Boryung. NextBioMedical climbed nearly 8% on strong export news, while Cellbion fell more than 13% as debate continued over the interpretation of its clinical trial results.
Pipeline momentum draws investor attention According to KG Zeroin MP Doctor (formerly MarketPoint), Curacle shares jumped 29.96% to close at 7,330 won, hitting the daily limit-up. The rally followed news that Curacle signed a strategic partnership with Boryung for the development of its diabetic nephropathy treatment candidate CU01, and a PharmEdaily report suggesting imminent technology export of MT-101 also boosted sentiment.
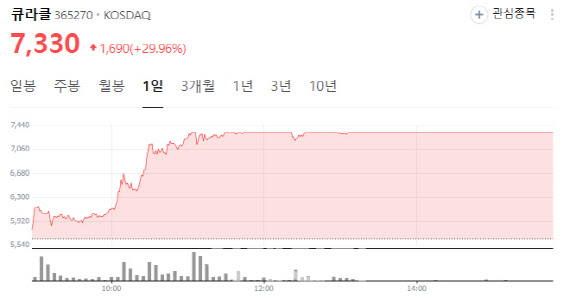 | | Curacle stock performance (Source: Naver Finance) |
|
Under the agreement, Curacle and Boryung will cooperate on strategies for Phase 3 trials, regulatory submissions, and joint review of licensing and commercialization options, including domestic and overseas rights. CU01 is in a Phase 2b trial in Korea with about 95% of patients already dosed, and completion is expected this year. A final clinical study report (CSR) due in January will serve as the basis for licensing discussions with Boryung.
The PharmEdaily report, first released as a paid article on Sept. 10 and made free on Sept. 11 at 9:30 a.m., also fueled trading. It revealed that Curacle had signed multiple material transfer agreements (MTAs) with overseas pharmaceutical companies for its kidney disease candidate MT-101. The article compared Curacle to Olix, which rebounded after inking a deal with Eli Lilly following a pipeline setback, and suggested Curacle could follow a similar trajectory as “the next Olix.” Analysts noted that competition among multiple global drugmakers signals a high probability of a license-out deal within the year.
Powder product takes flight NextBioMedical rose 7.98% to 78,500 won. Investor optimism grew on news of strong exports for its endoscopic hemostatic spray powder NexPowder.
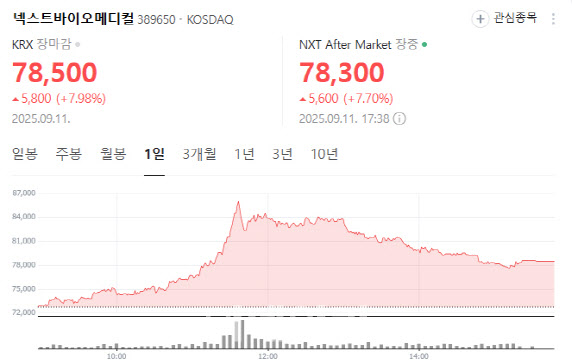 | | NextBioMedical stock performance (Source: Naver Finance) |
|
According to the Korea International Trade Association’s trade statistics service (TRASS), NexPowder recorded $810,000 in export value, or about 1.1 billion won, between Sept. 1 and 10. Extrapolated, that implies annual revenue of nearly 40 billion won, although seasonal factors and rollout schedules suggest actual sales could range from 20 to 30 billion won. Last year, NextBioMedical posted 9.5 billion won in revenue and an operating loss of 3.6 billion won.
A company official said, “It is difficult to attribute the stock move to a single factor, but sales expansion in the U.S. and Japan and clinical data supporting the product’s efficacy appear to be driving market interest.”
NexPowder was designated for reimbursement under Japan’s national health insurance on Sept. 5, with sales to begin this month. That comes just six months after approval from Japan’s Pharmaceuticals and Medical Devices Agency (PMDA). The product had already secured clearance from the U.S. Food and Drug Administration, Europe’s CE-MDR (Medical Device Regulation), and Korea’s Ministry of Food and Drug Safety. Global distribution is handled by Medtronic, the world’s largest medical device maker.
Clinical data debate weighs on shares Cellbion fell 13.14% to 20,500 won. The decline followed ongoing questions about the interpretation of topline data from a Phase 2 trial of its prostate cancer radiopharmaceutical candidate 177Lu-pocuvotide (Pocuvotide).
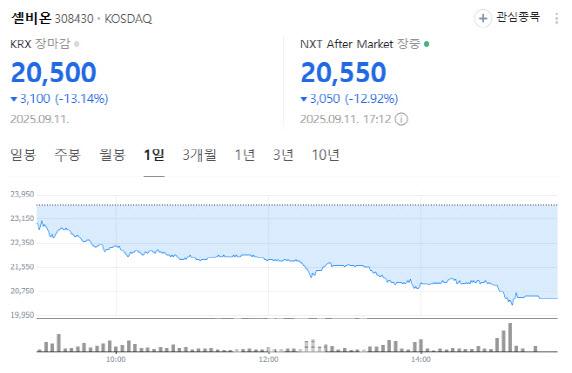 | | Cellbion stock performance (Source: Naver Finance) |
|
On Sept. 4, the company reported that among 91 patients with metastatic castration-resistant prostate cancer, the drug showed an objective response rate (ORR) of 35.9%, including complete responses in 8.97% and partial responses in 26.9%. Critics, however, pointed to a lower ORR compared with interim results, questions about comparing the data with Novartis’ Pluvicto, and limitations of using PSMA PET/CT imaging.
Cellbion countered that differences stem from varying sample sizes between interim and final analyses, and that the final dataset, processed under regulatory standards, is the one relevant for review. It emphasized that Pocuvotide outperformed Pluvicto’s Phase 2 Asian trial results (ORR 54.4% vs. 30%) and showed a lower adverse event rate (57.1% vs. 93.3%).
After plunging 21% on Sept. 5, Cellbion shares had stabilized with small moves through Sept. 8~10, before sliding again on Sept. 11. Trading volume spiked to more than 1.13 million shares, underscoring heavy selling pressure.
The company plans to file for conditional approval with Korea’s regulator after receiving the final CSR in the fourth quarter. A Cellbion official said, “We apologize for the confusion caused during the clinical announcement and will continue to provide transparent and accurate information to maintain shareholder trust.”