Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[Shin-Min Joon, Edaily Reporter] On June 17 shares of Shinpoong Pharm, HanmiScience, and D&D Pharmatech posted notable gains in the Korean biopharmaceutical stock market.
Shinpoong Pharm hit the daily upper limit following news that it had obtained a European patent for expanding the indication of its antimalarial drug Pyramax to include COVID19.
Hanmi Science, the holding company of the Hanmi Pharmaceutical Group saw its stock rise on investor expectations surrounding new research results on an innovative obesity drug under development by its affiliate Hanmi Pharmaceutical.
D&D Pharmatech shares continued their upward trend for a second day driven by the potential license out of its metabolic dysfunction associated steatohepatitis (MASH) treatment currently in development.
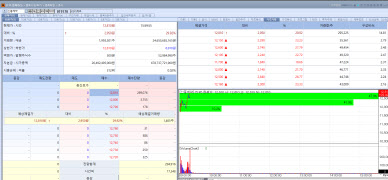 | | Shinpoong Pharm stock trend on June 17. (Image=MP Doctor) |
|
Shinpoong Pharm Hits Upper Limit on EU Patent for COVID19 Use of Antimalarial Drug
According to KG Zeroin’s MP Doctor platform, Shinpoong Pharm’s shares soared 29.92% to close at 12,810 won. The spike followed news that the company had secured a European patent related to the COVID19 indication of its antimalarial drug Pyramax.
Shinpoong announced that the European Patent Office (EPO) had granted a patent for the pharmaceutical composition of Pyramax for the prevention or treatment of epidemic RNA virus infections. This represents a formal patent grant following the previously pending application. The patent is expected to be officially published in the European Patent Bulletin on July 9.
The company has been actively expanding patent registrations for the COVID19 indication of Pyramax, having already secured patents in Africa and China. Domestically Shinpoong is also pursuing a patent registration for this indication and recently completed a Phase 3 clinical trial in South Korea.
Amid renewed concerns over a COVID19 resurgence in Asia, including in South Korea, the demand for effective therapeutics is rising. The Korea Disease Control and Prevention Agency (KDCA) forecasted a potential uptick in domestic COVID19 cases starting late June.
According to the World Health Organization (WHO) COVID19 cases have risen up to 9 percentage points over five weeks in regions including the Western Pacific, Southeast Asia and the Eastern Mediterranean. Neighboring countries like China, Thailand, and Taiwan are experiencing noticeable spikes.
In Korea the number of weekly hospitalizations has remained stable at around 100 over the past four weeks. However, the COVID19 detection rate, which had declined since March, began increasing around May 11~17 (week 20) hovering near 8%.
Last summer COVID19 hospitalizations rose from 456 in the last week of July to a peak of 1,441 by the third week of August. With neighboring Asian countries facing surges, health experts are warning against complacency in South Korea.
A Shinpoong representative commented “This patent recognizes the efficacy of Pyramax not only for COVID19 but also for other coronavirus respiratory infections such as SARS and MERS. The company is pursuing three major milestones: patent registrations, academic publications, and domestic regulatory approvals for the expanded indication.”
Hanmi Rises on Anticipation of Breakthrough Obesity Drug Data Shares of Hanmi Science climbed 16.14% to 43,900 won. As the holding company of Hanmi Pharmaceutical Group the stock benefitted from favorable developments across its affiliates.
Hanmi Pharmaceutical is set to unveil new obesity drug data at the American Diabetes Association (ADA) 2025 conference in Chicago which runs June 20~24. The company will present six preclinical and clinical studies on two next-generation treatments the triple acting agent HM15275 (targeting GLP, GIP, and LA-GLP) and the novel mechanism agent HM17321 (targeting UCN2).
Notably this will be the first disclosure of Phase 1 results for HM15275. Both compounds represent the next wave of Hanmi’s pipeline following its late stage asset efpeglenatide. HM15275 and HM17321 are being developed respectively as a best in class and first in class obesity treatment.
HM15275 is designed to minimize muscle loss while delivering over 25% weight reduction a level comparable to metabolic surgery. Phase 2 trials are expected to begin in the second half of this year focusing on sustained efficacy in reducing fat and improving lean mass in obese and metabolically ill patients.
HM17321 designed using Hanmi’s proprietary AI and structural modeling technology aims to reduce fat selectively while simultaneously increasing muscle mass an achievement previously thought physiologically unfeasible. The drug is positioned as a groundbreaking innovation with a novel mechanism.
Additionally Hanmi Fine Chemical is expanding its contract development and manufacturing (CDMO) business. The company is participating in the BIO International Convention 2025 in Boston (June 16~19) with its own booth. Hanmi Fine Chemical recently signed a contract with Ligachem BioScience (141080) to produce key intermediates for its antibody drug conjugate (ADC) platform ConjuALL.
A Hanmi Science official stated, “Investor enthusiasm is building ahead of the upcoming obesity drug data presentations.”
D&D Pharmatech Advances on License Out Prospects for MASH Drug Candidate D&D Pharmatech shares rose 7.17% to close at 136,100 won, extending their rally to a second day. The stock gained momentum on expectations of an out licensing deal for DD01, its lead candidate for metabolic dysfunction-associated steatohepatitis (MASH).
DD01 recently achieved statistically significant results in a U.S. Phase 2 trial’s primary endpoint. Impressively 75.8% (25 out of 33) of patients in the treatment arm experienced a ≥30% reduction in liver fat, compared to just 11.8% in the placebo group (p<0.0001). At week 12, the average liver fat reduction in the treatment group was 62.3%, versus 8.3% for placebo showing a rapid and robust effect.
Notably DD01’s efficacy after 12 weeks was comparable to that of Boehringer Ingelheim’s survodutide a glucagon dual agonist which demonstrated similar liver fat reduction over a 48 week period.
D&D Pharmatech recently signed a consulting agreement with a leading U.S. investment bank to explore out licensing and global partnerships for DD01. The company plans to actively pursue deals based on the favorable Phase 2 data.
Recent MASH licensing deals include OliX’s February agreement with Eli Lilly worth 911.7 billion won for RNAi based candidates and GSK’s May acquisition of Boston Pharmaceuticals’ efruxifermin for 3.7 trillion won.
A D&D Pharmatech spokesperson said “We aim to maximize the value of our promising clinical data through our collaboration with the newly retained investment bank and will make every effort to secure a major licensing deal.”



![Xcell Therapeutics Hits Upper Limit...Celemics·QuadMedicine ↑[K-Bio Pulse]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/02/PS26020600366b.jpg)


![문여정 IMM인베스트먼트 전무 "ADC·DDS·CNS 주목"[바이오 VC 집중조명⑩]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/02/PS26020600489b.jpg)
![인도, 글로벌 저가 제네릭에서 바이오 허브로 탈바꿈[제약·바이오 해외토픽]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/02/PS26020700379b.jpg)

