Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[Yu Jin-hee, Edaily Reporter] On Oct. 28, Korea’s stock market saw strong gains in the bio and healthcare space (drugmakers, biotechs and medical-device makers). Three names landed in the day’s top-10 gainers, underscoring renewed interest in the sector. In particular, 50-year-old Samik Pharmaceutical’s successful listing signaled a potential revival in biotech sentiment.
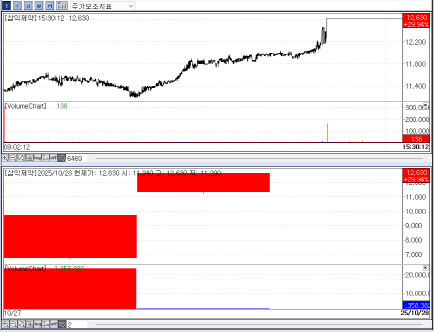 | | Recent stock price trend of Samik Pharm. (Source: KG Zeroin MP Doctor |
|
Samik Pharm shows balance of stability, profitability and growth According to KG Zeroin’s MP Doctor (formerly MarketPoint), the day’s top-10 gainers included Samik Pharmaceutical, P&S Robotics and Bistos. They rose 29.94% (closing at 12,630 won), 18.40% (213,000 won) and 16.53% (1,713 won), respectively.
The standout was Samik Pharmaceutical, which entered Kosdaq on Oct. 27 via a merger with Hana Financial No. 28 SPAC. The company is known for over-the-counter brands such as the cold remedy “Maparam,” the motion-sickness syrup “Novomin,” and the pediatric line “Kiddy.” Shares hit the daily upper limit for two straight sessions on Oct. 27 and 28, highlighting the latent strength of a traditional drugmaker often seen as undervalued within the broader biotech complex.
Analysts say investor appetite was helped by Samik’s balanced profile across stability, profitability and growth potential. Founded in 1973, the company has focused on fundamentals and steady expansion. Despite a tough market, it delivered double-digit annual growth over the past three years and posted a record 55.9 billion won in sales last year.
Leverage-often a weak point for biotechs-is low: the debt ratio stood at 23.8% at end-2024. Governance is also stable. CEO Lee Chung-hwan is the largest shareholder with 27.97%; founder Chairman Lee Se-young holds 14.92%, bringing related-party ownership to 81.32%.
Samik also has new-drug optionality. Its lead pipeline is SIKD1977 for post-herpetic neuralgia (PHN). The company is running a domestic Phase 2 trial, exploring add-on use with current standard treatments and aiming to address unmet needs such as therapy for older patients with an emphasis on safety. Industry estimates put the global PHN-drug market at about $1.76 billion in 2024, rising to roughly $2.5 billion by 2033.
“The proceeds from our Kosdaq listing will go to expanding manufacturing capacity and R&D,” a Samik official said. “We will particularly focus investment on our lead pipeline SIKD1977 to accelerate early results.”
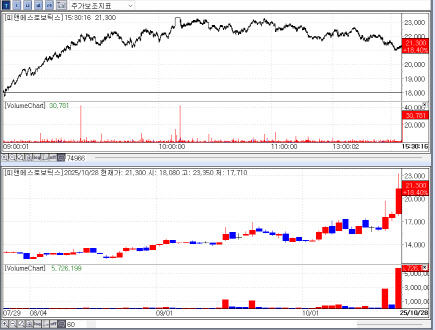 | | Recent stock price trend of P&S Robotics. (Source: KG Zeroin MP Doctor |
|
P&S Robotics rallies as investors favor “stability” P&S Robotics, a medical rehabilitation robot specialist, appeared to benefit from the same broad preference for stability. In a rising market, investors have gravitated toward names perceived as relatively stable.
The immediate catalyst was a bonus issue announced on Oct. 24: one new share for each common share. The company will issue 6,565,740 new shares; the record date is Nov. 10 and the listing date is Dec. 1. Shares rose for three straight sessions after the disclosure.
A bonus issue converts retained earnings into capital and allocates new shares to existing holders, often read as a signal of balance-sheet strength. It does not, however, inherently raise enterprise value, and stocks frequently give back gains around the issuance. Even so, P&S Robotics could prove an exception. The company develops advanced humanoid and service robots alongside rehabilitation gait robots and is layering in artificial intelligence-supporting a constructive growth outlook. In the first half, revenue and operating profit were 4.7 billion won and 1.5 billion won, up 78.8% and 213.5% year on year. Korea Investment & Securities projects P&S will top 10 billion won in annual revenue this year with about 4 billion won in operating profit.
Elsewhere, Bistos’ gains appeared linked to recent positives such as obtaining Medical Device Single Audit Program (MDSAP) certification. Bistos focuses on vital-sign monitors, fetal/neonatal monitors and electrocardiographs. Beyond domestic hospitals, it has secured customers in more than 100 countries and is targeting further scale-up as a global med-tech player.




![Xcell Therapeutics Hits Upper Limit...Celemics·QuadMedicine ↑[K-Bio Pulse]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/02/PS26020600366b.jpg)






