Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[Kim Jinsoo, Edaily Reporter] Shares of South Korean health-care names S-Biomedics, Geninus and HansBiomed surged around 15% on Thursday, with S-Biomedics climbing after saying its Parkinson’s stem cell therapy can move straight into a U.S. Phase 3 trial based on Korean data.
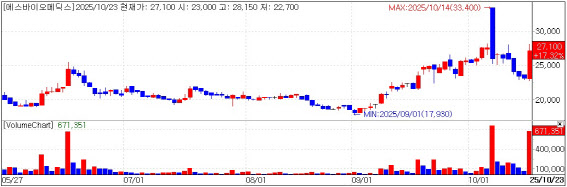 | | S-Biomedics stock trend. (KG Zeroin’s MP Doctor) |
|
S-Biomedics Parkinson’s treatment set for U.S. FDA Phase 3 S-Biomedics closed up 16.02% at 26,800 won, according to KG Zeroin’s MP Doctor. The company said it was told in a pre-IND (pre-investigational new drug) meeting that results from its Korean Phase 1/2a study of “TED-A9” are sufficient to start a Phase 3 trial in the United States without running a separate U.S. early-stage study.
TED-A9 entered Phase 1/2a in 2023, completed patient dosing in February 2024, and in April reported one-year follow-up data. In MDS-UPDRS Part 3, a motor function scale for Parkinson’s disease, the low-dose cohort improved by an average 21.8% and the high-dose cohort by 26.9%. On the Non-Motor Symptoms Scale (NMSS), cohorts improved 29.2% and 32.8%, respectively, and imaging showed stronger dopaminergic cell engraftment signals in the high-dose group, the company said.
An S-Biomedics official said the FDA can authorize pivotal trials using foreign early-stage data “if safety and efficacy are sufficiently demonstrated,” adding that the agency’s flexibility contrasts with some markets that require domestic early-phase data.
The company also said a separate RMAT designation or other expedited program is not required to begin a commercial (pivotal) trial, noting those programs accelerate review but are not prerequisites. S-Biomedics plans to file a formal Phase 3 IND with the FDA “soon.”
Geninus up 15% on business transformation plans Geninus rose 14.74% to 2,195 won on expectations it will pivot from diagnostics to an AI- and genomics-driven drug-discovery platform.
A Daishin Securities report said Geninus is shifting away from hospital-dependent NGS testing, where pricing pressure has weighed on margins, and will instead monetize its datasets and tools to support pharmaceutical clinical decision-making.
Revenue declined from 10.1 billion won in 2022 to 7.0 billion won in 2023 and 6.5 billion won last year, while operating losses widened to 12.3 billion won. Analyst Han Song-hyup wrote that Geninus has assembled about 400 million spatial transcriptomics data points and single-cell data from 4,000 patients, calling the trove a key advantage and arguing the company could be re-rated as a high-growth data platform.
A company official said investor attention was sparked by the brokerage note and that there were “no other special issues.”
HansBiomed soars 47% this week HansBiomed jumped 16.35% to 33,800 won, hitting an intraday 52-week high of 35,800 won. The stock has leapt 46.96% since Oct. 20, driven by early traction for its extracellular matrix (ECM)–based skin booster.
The company late last month launched “Cellerdiem”, which uses human acellular dermal matrix (hADM) to directly replenish collagen. Word-of-mouth that ECM-based boosters outperform PDRN/PN-based products has lifted sales, the company said.
HansBiomed projects October sales of about 13,000 units and estimates December could reach 22,000 units. On that run-rate, it raised its 2025 revenue target for the product to 25–30 billion won from 3–4 billion won.
“Production capacity is essentially matched to orders, and we are running the plant at maximum to meet demand,” a company official said.








![인도, 글로벌 저가 제네릭에서 바이오 허브로 탈바꿈[제약·바이오 해외토픽]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/02/PS26020700379b.jpg)