Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[Song Young-doo, Edaily Reporter] Shares of Aptabio Co. surged Thursday on investor optimism that the company could follow a path similar to that of Gilead Sciences, evolving from a biotech startup into a global pharmaceutical giant. Onconic Therapeutics Inc. also posted gains on prospects for milestone payments related to a licensing deal in China. In contrast, shares of Bukwang Pharm Co. tumbled after it announced a 100 billion won rights offering.
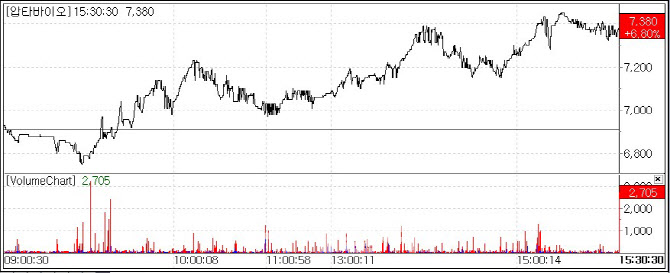 | | Aptabio stock trend (Source=MP Doctor by KG Zeroin) |
|
According to MP Doctor, formerly MarketPoint, by KG Zeroin, Aptabio shares closed up 6.8%, at 7,380 won. It marked the second time since March 18 that the stock has posted a daily gain of more than 6% over the past 10 trading sessions.
Although Aptabio shares opened lower, they reversed course after a PharmEdaily pay-to-read article part of Edaily’s premium pharma-biotech content(
“From Bio Venture to Korea‘s Gilead, The Rise of Aptabio”[Inside Aptabio, Part ①]) was published at 9:30 a.m. on a major online portal. “There had been barely any trading volume, but demand surged after the article went live” a company official said.
In the interview, Aptabio CEO Soo-Jin Lee voiced her ambition to grow the company into a global developer of innovative drugs, much like Gilead.
Gilead, founded in 1987 as Oligogen, made its mark by pursuing antiviral drug research that other major pharmaceutical firms had avoided. The company developed Tamiflu in 1999 and saw explosive sales during the 2009 swine flu pandemic. It later launched blockbusters such as HIV treatments and the hepatitis C drug Harvoni. As of 2023, Gilead posted $26.4 billion in revenue, ranking No. 15 among global pharmaceutical companies.
Aptabio is currently developing first-in-class therapies using proprietary aptamer-drug conjugate technology and NOX enzyme inhibitors. Only two companies are pursuing drugs based on NOX inhibition Aptabio and a Swedish biotech acquired by a Japanese firm.
“NOX enzymes are embedded in the cell membrane,” Lee said. “They transmit external signals into the cell and activate proteins that can damage tissue.”
Aptabio has attracted the attention of Merck, which is collaborating on a combination therapy involving Aptabio’s APX-343A and Merck’s blockbuster immunotherapy Keytruda. In December, Aptabio submitted a Phase 1 clinical trial application to South Korea’s Ministry of Food and Drug Safety. APX-343A is designed to inhibit cancer-associated fibroblasts in the tumor microenvironment and boost immune cell infiltration, thereby enhancing anti-cancer effects. If the combination proves successful, Aptabio could replicate the revenue-generating model of Yuhan Corp.’s cancer drug Lazertinib.
Onconic Soars on Milestone Hopes, FDA Orphan Drug StatusOnconic Therapeutics led gains among South Korean biotech stocks, closing up 12.8%. The stock approached the 20,000won level for the first time in nearly three months.
Investors were buoyed by a combination of factors, including possible milestone payments from a licensing deal and optimism over Onconic’s cancer drug candidate, Nesuparib, which recently received U.S. Food and Drug Administration orphan drug designation. The company also plans to present research at next month’s American Association for Cancer Research (AACR) conference.
On March 24, Onconic disclosed that it had invoiced a milestone payment for Zacubo, a gastroesophageal reflux disease treatment designated Korea’s 27th novel drug. In March 2023, the company licensed Zacubo to China’s Livzon Pharmaceutical Group Co., granting exclusive rights for development, approval, production and commercialization in Greater China. The total deal value was $127.5 million, including a $15 million upfront payment.
Onconic previously received a $3 million milestone payment from Livzon and has now billed an additional $1.5 million for completing the commercial-scale manufacturing tech transfer. The latest payment represents roughly 15% of the company’s 2024 revenue target of 14.8 billion won.
Nesuparib, aimed at gastric and gastroesophageal junction cancers, was recently granted FDA orphan drug status, which offers benefits including expedited review and potential conditional approval. The company is scheduled to present study results at the AACR conference in April.
“Investor expectations have grown around both the Zacubo milestones and Nesuparib’s designation,” an Onconic spokesperson said. “Since new biologic drugs are less impacted by export tariffs and we’re generating earnings through proprietary development, the market is viewing us favorably.”
Bukwang Slumps on $75M Rights Offering Despite Growth PlanBukwang Pharm shares fell 13.3%, to close at 3,900 won after the company’s board approved a 100 billion won rights offering. The stock hit a 52week low of 3,855 won earlier in the day.
The rights offering will be conducted via shareholder allocation followed by a public subscription. The company plans to issue 30.2 million new shares at 3,310 won per share. Proceeds will be used for facility expansion(84.5 billion won) and working capital(15.5 billion won).
Bukwang said the investment is essential for boosting its performance and achieving long-term growth. Plans include acquiring and upgrading manufacturing facilities, entering the contract development and manufacturing organization (CDMO) market, and expanding its drug pipeline.
The company will remodel its ointment and liquid formulation production lines in Phase 1, increasing capacity from 200 million to 400 million tablets annually with a 20 billion won investment. In Phase 2, it will invest an additional 30 billion won to either expand capacity or acquire new facilities based on market conditions.
Bukwang also plans to pursue CDMO opportunities and improve its drug portfolio by enhancing existing formulations, in-licensing projects, conducting small-molecule drug research, and developing additional indications for Latuda, including major depressive disorder.
The company aims to become a top-20 pharmaceutical firm in Korea with annual revenue of 400 billion to 500 billion won by 2030. “To take the next step, we need to generate more substantial revenue” a Bukwang representative said. “Without investment, we can’t break through our current limitations. Despite running our facilities at full capacity, we’ve experienced product shortages. Expansion and new drug development will be the foundation for growth through 2030.”





!['공매도+관세폭탄+암학회 '...4월,최고 난이도에 예측불허[월간 바이오 맥짚기]](https://image.edaily.co.kr/images/vision/files/NP/S/2025/04/PS25040100510b.jpg)

![온코닉테라퓨틱스, 표적항암제 임상 유효성 입증에 上...메지온도 상승[바이오맥짚기]](https://image.edaily.co.kr/images/vision/files/NP/S/2025/04/PS25040100379b.jpg)


