Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[Kim Jinsoo, Edaily Reporter] Shares of ABL Bio soared to the daily limit Friday after the company announced a 4 trillion won licensing deal with global pharmaceutical giant GSK. Meanwhile, Dt&CRO extended its rally, driven by speculation tied to upcoming presidential elections.
In contrast, medical AI companies JLK and VUNO hit 52-week lows amid ongoing uncertainty, including concerns over potential U.S. tariffs.
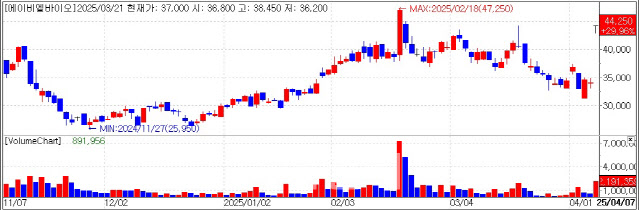 | | Recent Stock Performance of ABL Bio. (KG Zeroin’s MP Doctor) |
|
ABL Bio Soars on $3 Billion License-Out Deal with GSK According to KG Zeroin’s MP Doctor platform (formerly Market Point), ABL Bio shares surged from market open and closed at the daily upper limit, jumping 10,200 won to 44,250 won.
ABL Bio said it signed a license-out agreement with GSK covering its blood-brain barrier (BBB)-penetrating platform Grabody-B. The deal is worth a total of 2.14 billion British pounds ($2.7 billion or 4.1 trillion won).
Under the agreement, ABL Bio will receive £38.5 million ($48.7 million or 73.9 billion won) in upfront payments and £38.6 million in near-term milestones. Additional payments tied to development, regulatory, and commercialization milestones across multiple programs will follow.
Excluding returned or canceled deals, this is the second-largest licensing agreement in South Korea’s biotech sector, following Alteogen’s 2020 deal with Merck & Co. (MSD).
Grabody-B is a platform designed to help drugs cross the BBB, which acts as a defense shield against harmful substances but also blocks therapeutic agents. Grabody-B uses insulin-like growth factor 1 receptor (IGF1R) as a transport mechanism to deliver drugs into the brain.
Under the agreement, the companies aim to develop multiple oligonucleotide- or polynucleotide-based therapies including siRNA and ASO drugs. ABL Bio will provide the core technology and know-how, while GSK takes charge of preclinical and clinical development, manufacturing, and commercialization.
With this deal, ABL Bio’s cumulative license-out volume approaches 7 trillion won. Since 2018, the company has signed major licensing deals with Yuhan Corp. (59 billion won), Compass Therapeutics ($410 million in 2018 and $185 million in 2021), SystImmune ($363.5 million), and Sanofi ($1.06 billion in 2022).
“This collaboration is expected to address unmet needs for patients suffering from degenerative brain diseases,” an ABL Bio official said.
Dt&CRO Shares Climb on Political Speculation Dt&CRO’s share price jumped 20.51% to close at 8,930 won, continuing gains from the previous session when it hit the daily limit. The rally is attributed to the stock being labeled a “political theme stock” ahead of the presidential election.
The company is often linked to candidate Han Dong-hoon, as independent board director Lee Sung-kyu shares an academic background with Han—both graduated from Seoul National University School of Law and Columbia Law School in the U.S.
Dt&CRO positions itself as Korea’s first full-service CRO, covering everything from preclinical to clinical development. It offers services such as GLP toxicology testing, pharmacokinetics/pharmacodynamics (PK/PD), efficacy evaluation, analytical testing, bioequivalence studies, clinical trials, and regulatory consulting for FDA and EMA approvals.
The company said there were “no specific issues related to core operations” affecting recent share movement.
Medical AI Firms JLK, VUNO Hit Lows on Tariff Jitters Medical AI firms JLK and VUNO both saw their shares tumble to new 52-week lows. JLK closed at 5,970 won, down 9.82%, after falling as low as 5,960 won during the session. VUNO dropped 11.01% to close at 16,240 won, with an intraday low of 16,190 won.
The drop is largely attributed to concerns over potential U.S. tariffs. While JLK and VUNO’s AI solutions are categorized as software, not hardware, there is growing worry they could be affected by future customs decisions.
JLK has secured 510(k) FDA clearances for seven AI-based solutions, including MEDIHUB Prostate, JBS-LVO, JLK-CTP, JLK-PWI, JLK-ICH, JLK-ailink, and SDH. The company is currently focused on establishing early-stage supply chain partnerships with hospitals in key regions.
“The recent market downturn reflects broader external conditions rather than internal fundamentals,” a JLK official said.
VUNO is expected to receive U.S. Food and Drug Administration (FDA) clearance for its cardiac arrest prediction solution, VUNO Med DeepCARS, in the first half of this year. The company plans to turn profitable through sales of the AI-powered device but may need to revise its strategy depending on future U.S. tariff decisions.
“The recent drop in our stock price reflects turbulence in domestic and global markets,” a VUNO spokesperson said. “However, our flagship solutions are making visible progress in overseas markets, and regulatory processes that were previously stalled due to organizational changes at the FDA are now moving forward as a dedicated officer has been assigned.”
The company added that it expects its share price to recover as regulatory progress accelerates and product rollout overseas gains traction.





![LG 출신 김현수 대표, 독보적 광학 기술로 글로벌시장 접수[휴비츠 대해부①]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/03/PS26030800061b.jpg)




