Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[Kim Jinsoo, Edaily Reporter] On Aug. 8 South Korea’s biotech market drew investor attention as Curiox Biosystems confirmed a collaboration with a global contract research organization (CRO).
Excel Therapeutics rose on government support for its artificial blood project while HK inno.N advanced on positive U.S. clinical trial data for its gastroesophageal reflux disease drug, K-CAB.
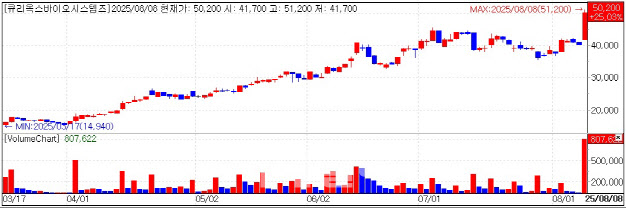 | | Curiox Stock. (KG Zeroin) |
|
Curiox Formalizes Global CRO Partnership According to KG Zeroin MP Doctor (formerly MarketPoint), Curiox shares closed at 50,200 won, up 25.03%. Intraday the stock gained more than 27% to hit a 52week high.
The rally came after U.S. based Charles River Laboratories, a leading CRO, publicly confirmed its collaboration with Curiox. Headquartered in Massachusetts, Charles River offers integrated services in preclinical research, laboratory animal science, and safety assessments. The company reported $4.1 billion in revenue last year, operates over 80 facilities worldwide, and is regarded as a trusted partner in the life sciences industry.
On its “Eureka” online content platform, Charles River wrote: “By combining Charles River’s CRO service expertise with Curiox’s innovation in non-centrifugal sample handling, we are creating a new standard for translational strategy.”
It added: “A strong automation infrastructure is essential to shift from animal testing to non-animal new approach methodology (NAM) workflows. Curiox’s C-FREE Pluto system offers both operational efficiency and cost savings.”
The statement indicates Charles River has formally adopted Curiox’s technology and equipment in its automation related strategy.
The “C-FREE” technology developed under CEO Namyong Kim, fully automates cell analysis sample preparation eliminating the centrifugation step a key bottleneck in automating cell washing. By doing so it minimizes cell loss and deformation reduces time and cost, and improves data accuracy and reproducibility.
A Curiox official said “Eureka is operated directly by Charles River. The post appears to have been made in the context of collaboration, which likely influenced the stock price.”
Excel Therapeutics Gains on Artificial Blood Classification Excel Therapeutics shares rose as much as 28.14% during the session before ending up 9.91% at 3,495 won. The company is viewed as a beneficiary of the Ministry of Food and Drug Safety’s (MFDS) decision to classify cell-based artificial blood as an advanced biopharmaceutical.
The MFDS announced the classification on Aug. 8 under the Act on Safety and Support for Advanced Regenerative Medicine and Advanced Biopharmaceuticals. Cell-based artificial blood refers to red blood cells and platelets produced from stem cells to address potential blood shortages.
Since 2023, the MFDS has been running the “Cell-Based Artificial Blood Manufacturing and Demonstration Platform Technology Development Project” to develop technologies for producing red blood cells and platelets from stem cells and to commercialize them.
Excel Therapeutics, the lead contractor for the government project, is developing culture media for artificial blood. The company plans to invest about 47 billion won by 2027 to develop cell differentiation and proliferation technologies and produce 5~10㎖ of artificial red blood cells and platelets. It will also establish standardized manufacturing processes, quality control standards, and testing methods.
An Excel official said “Multiple ministries are focusing heavily on this project. The recent share price gain seems to reflect increased attention to artificial blood related developments.”
HK inno.N Eyes Blockbuster Status HK inno.N shares jumped as much as 18% in early trading to 53,100 won, a 52-week high, before trimming gains to close up 2.44% at 46,100 won. The rally followed positive Phase 3 trial results in the United States for its K-CAB (tegoprazan) therapy for gastroesophageal reflux disease (GERD).
The company said its U.S. partner, Sebela Pharmaceuticals, announced topline results from the TRIUMpH study, a Phase 3 trial evaluating maintenance therapy after healing of erosive esophagitis (EE). The 24 week maintenance rate for all patients (LA grades A~D) showed non inferiority and statistical superiority of all tegoprazan dose groups compared with lansoprazole.
Based on the results, Sebela plans to submit a New Drug Application (NDA) to the U.S. Food and Drug Administration in the fourth quarter of this year for EE and non erosive GERD indications.
Analysts say K-CAB has strong potential to become a blockbuster a drug with annual sales exceeding 1 trillion won given that the global peptic ulcer drug market is worth about 20 trillion won, with the U.S. accounting for roughly 4 trillion won.
K-CAB is already approved in 54 countries, including the U.S. and China, and is marketed in nine. The drug could reach blockbuster status within two years.
An HK inno.N spokesperson said “Sebela holds all U.S. rights. We cannot comment beyond the planned NDA submission in the fourth quarter, and the U.S. launch timing will depend on FDA review.”



![Biodefense Bill, Abortion Pill Push Boost Market; Genomictree Climbs[K-Bio Pulse]](https://image.edaily.co.kr/images/vision/files/NP/S/2025/08/PS25081300527b.jpg)


![셀트리온, 합병 후 분기 최대 실적…“짐펜트라 주춤, 하반기 마케팅 관건”[인베스트 바이오]](https://image.edaily.co.kr/images/vision/files/NP/S/2025/08/PS25081000170b.jpg)
![[美 꽂힌 바이오]라파스 “화장품 사업으로 마이크로니들 대량생산 능력 입증”](https://image.edaily.co.kr/images/vision/files/NP/S/2025/08/PS25081300619b.jpg)


![‘백기사’ 형인우 대표, 73억 투입해 엔솔바이오 주가 하락 제동[화제의 바이오人]](https://image.edaily.co.kr/images/vision/files/NP/S/2025/08/PS25081000401b.jpg)