Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[Seok Ji-hoen, Edaily reporter] Shares of Selbion gained for a second straight day Wednesday as investors responded positively to the company’s combination trial strategy involving global pharmaceutical giant Merck(MSD). Dongkook Life Science also surged following news of a 100% stock dividend, while Ildong Pharmaceutical ended the session up more than 7% on renewed expectations for its oral GLP-1 drug candidate.
“Potential to Outperform Lekraza” According to KG Zeroin’s MP Doctor (formerly Market Point), shares of Selbion closed at 27,450 won, up 1,950 won or 7.65%. The stock rally followed a recent interview in which CEO Kwon Kim unveiled the company’s plan to co-develop a prostate cancer treatment in combination with Merck’s immunotherapy Keytruda.
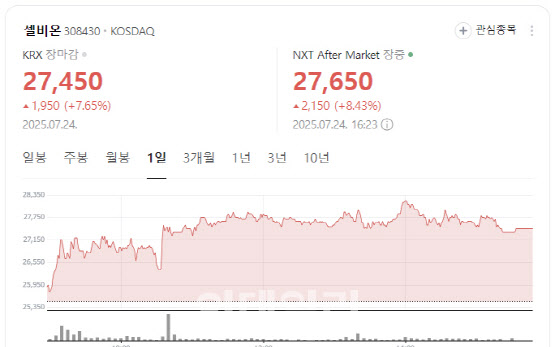 | | Selbion stock performance (Image captured from Naver Finance) |
|
PharmEdaily had published a paywalled interview with Kim on July 21 under the headline “Selbion CEO: ‘Keytruda Combo Could Beat Lekraza’.” The article was made publicly available on July 23. Selbion’s shares rose 5.6% that day and continued their climb on Wednesday.
In the interview, Kim said, “If our proprietary radiopharmaceutical Lu-177-DGUL proves successful in combination with Keytruda, it has the potential to outperform Lekraza from Yuhan Corp. We are currently in positive talks with Merck.”
Selbion has applied for a Phase 1 clinical trial in Korea for the combination therapy. A separate Phase 2 monotherapy trial has completed patient dosing, with topline data expected in the third quarter. The company plans to apply for conditional approval later this year and is targeting a domestic launch in the first half of 2026. Industry analysts forecast the treatment could generate up to 20 billion won ($15 million) in domestic revenue once on the market.
What sets Lu-177-DGUL apart is its ability to convert prostate cancerㅡa so-called “cold tumor” with poor immune responseㅡinto a “hot tumor,” thereby enhancing the efficacy of immunotherapies such as Keytruda. This could allow the therapy to overcome limitations seen with Keytruda monotherapy in prostate cancer.
According to market research, the global prostate cancer treatment market is expected to grow from 21 trillion won ($15.2 billion) in 2022 to 40 trillion won ($29 billion) by 2030. “We are also in talks with other global pharmaceutical companies regarding licensing and co-development,” Kim added. “These discussions will accelerate our monetization and pipeline expansion as Lu-177-DGUL approaches commercialization.”
When asked about the stock move, a Selbion spokesperson told Edaily, “There is no specific development within the company to share at this time.”
100% Stock Dividend Boosts Dongkook Life Science Shares of contrast agent specialist Dongkook Life Science closed at 9,660 won, up 620 won or 6.86%, after the company announced a 100% stock dividend.
Dongkook’s board of directors approved the plan to issue one new share for each existing common share. The company said the move aims to expand liquidity and reinforce shareholder-friendly policies, backed by strong fundamentals.
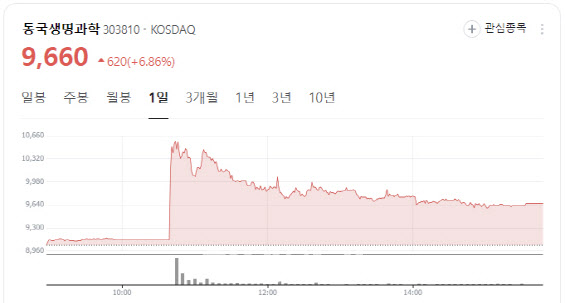 | | Dongkook Life Science stock performance (Image captured from Naver Finance) |
|
As a result, Dongkook’s total number of outstanding shares will double from 15.99 million to 31.98 million. The record date for the stock dividend is Aug. 8, with listing of the new shares scheduled for Aug. 29. Capital stock will rise from 8 billion won to 16 billion won.
“The purpose of this decision is to maximize shareholder value and broaden the investor base,” a company spokesperson said. Since its establishment in 2017, Dongkook has posted average annual growth rates of 15.6% in revenue and 14.5% in operating profit.
The company operates a fully integrated vertical manufacturing system for contrast agents and is currently investing in new business areas such as an MRI contrast agent, a tripling of capacity at its Anseong plant, and contract manufacturing (CMO). Sales of the company’s latest contrast agent, Mediray, have been strong since its launch in late 2024, supporting further earnings momentum.
“We don’t have any additional comment regarding the stock movement,” a company official said. “The dividend is purely aimed at enhancing shareholder returns and stock liquidity.”
“Competitive With Global Candidates” Shares of Ildong Pharmaceutical closed at 22,150 won, up 1,550 won or 7.52%, amid growing optimism over its oral GLP-1 obesity treatment candidate.
According to a July 24 report by IV Research, Ildong’s oral small-molecule GLP-1 drug ID110521156 has produced clinical data comparable to global competitors and could warrant a revaluation of the company’s pipeline assets.
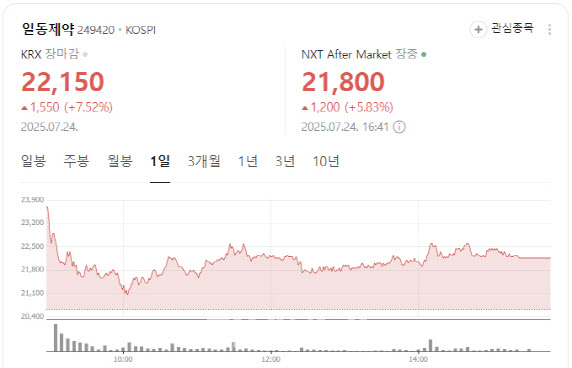 | | Ildong Pharmaceutical stock performance (Image captured from Naver Finance) |
|
The report noted that no clear winner has emerged between peptide-based and small-molecule oral GLP-1 drugs. While peptides currently dominate, small molecules offer advantages such as better tissue permeability, higher bioavailability, and lower manufacturing costs.
Eli Lilly’s small-molecule GLP-1 candidate Orforglipron showed 7.9% weight loss in its Phase 3 ACHIEVE-1 trial, but up to 8% of participants discontinued due to gastrointestinal issues. Pfizer, meanwhile, halted development of Lotiglipron and Danuglipron due to liver toxicity concerns.
By contrast, Ildong’s ID110521156 showed strong safety and efficacy in its domestic Phase 1 trial. The low-dose (50mg) group saw average weight loss of 5.50%, and the mid-dose (100mg) group recorded 6.89% (max 11.9%), versus 2.72% in the placebo group. No liver toxicity or treatment-related discontinuations were reported.
“These results are competitive with Orforglipron, and the upcoming topline data for the high-dose (200mg) cohort, expected in August?September 2025, will be a key catalyst,” IV Research stated. The trial was designed with input from a leading global obesity drug developer, and licensing talks are reportedly underway.
On the financial front, IV Research noted that Ildong recorded net losses from 2020 due to heavy R&D investment, but returned to operating profit in 2024. The company’s R&D expenses dropped from 127.6 billion won in 2022 (20% of revenue) to 46.3 billion won in 2024 (7.5%).
“With clinical progress becoming visible, now is the time to reflect the value of the company’s GLP-1 pipeline in its market capitalization,” the report concluded.
When asked for comment, an Ildong official told Edaily, “There’s no specific internal development that we can share regarding the stock price movement.”





![Selbion Up 2 Days, Eyes Lekraza Upside<BR><BR>[[K-Bio Pulse]](https://image.edaily.co.kr/images/vision/files/NP/S/2025/07/PS25072500367b.jpg)


!["'렉라자' 뛰어넘을 자신" 셀비온, 연이틀 상승[바이오맥짚기]](https://image.edaily.co.kr/images/vision/files/NP/S/2025/07/PS25072500558b.jpg)
![美·中 혁신 신약 승인 전망은?[제약·바이오 해외토픽]](https://image.edaily.co.kr/images/vision/files/NP/S/2025/07/PS25072600146b.jpg)

