Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[Song Young-doo, Edaily Reporter]Anigen’s stock which had been on a downward trend for the past five trading days rebounded sharply. The stock’s recent decline was reversed as optimism surrounding the potential approval of its U.S. cGMP(Current Good Manufacturing Practice) certification resurfaced. Meanwhile S-Biomedics also saw another rise in its stock price, fueled by expectations for its stem cell-based Parkinson’s treatment. In contrast, Lunit, despite announcing a major supply contract for its AI solution with Saudi Arabia, experienced a sharp drop in its stock price.
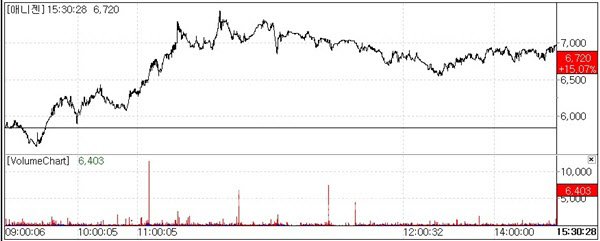 | | Anigen stock trend.(Source: KG Zeroin MP Doctor) |
|
Anigen Soars Over 15% on U.S. cGMP Approval ProspectsAccording to KG Zeroin MP Doctor(formerly Market Point) on the 6th, Anigen surged 15.07%(880 won) to close at 6,720 won, surpassing the 6,000 won mark. After peaking at 9,150 won on February 20, the stock had declined for six out of the following eight trading sessions, dropping to 5,840 won on the 5th. However, it made a strong rebound on this day.
The company stated that there were no specific new developments driving the stock price increase. However, market analysts suggest that recent pre-approval inspections conducted by the U.S. Food and Drug Administration(FDA) for cGMP certification at Anigen’s active pharmaceutical ingredient(API) manufacturing facility, along with positive feedback received, have reignited expectations for U.S. market entry and exports.
Anigen’s representative commented “Despite the lack of negative news, the stock had been declining for consecutive days. We view this as a technical rebound, but the anticipation surrounding the U.S. cGMP approval for our API production facility also likely contributed.”
If Anigen secures cGMP approval from the FDA, it will be able to immediately export its key active pharmaceutical ingredients(API), Leuprorelin and Ganirelix, from its Peptide Farm facility in Osong to the U.S. Both are core products for the company. Leuprorelin is used to treat infertility, prostate cancer, endometriosis, and precocious puberty, while Ganirelix is primarily used for infertility treatment.
Both are G-protein-coupled receptor(GPCR) modulating peptides, and Anigen is the only domestic producer.
The U.S. market for these products is estimated to be worth $5.8 billion. Anigen has already secured inventory ready for immediate export. Furthermore, it has reportedly received an initial order for Leuprorelin API from a U.S. client. CEO Kim Jae-il stated that once U.S. exports begin in earnest, the company could supply up to 50 kg annually, leading to a significant revenue jump.
Anigen posted revenue of 10.3 billion KRW in 2021, 8.7 billion won in 2022, and 5.6 billion won in 2023, marking a steady decline over the past three years.
Anigen’s representative clarified, “The initial order from the U.S. client is for sample testing and not a large volume. However, once we secure cGMP approval, we can immediately export our inventory.”
S-Biomedics Gains on Parkinson’s Treatment and Regulatory MomentumS-Biomedics, which is developing a stem cell-based Parkinson’s disease treatment, saw the biggest stock increase among biotech firms. Its stock closed at 24,000 won, up 7.62%(1,700 won) from the previous day.
Since attending the JP Morgan Healthcare Conference in San Francisco this January, the company’s stock has been on a steady uptrend. It climbed from 17,290 won on January 2 to 21,450 won by January 13, during the conference. On February 20, it peaked at 26,500 won, then fluctuated before resuming its upward trend on March 5.
S-Biomedics is set to announce the final results of its Phase 1 clinical trial for its Parkinson’s treatment ‘TED-A9’ in April. Early results from both low-dose and high-dose groups have shown higher efficacy than a competing treatment by BlueRock Therapeutics, raising expectations for potential licensing deals. The company is also considering directly progressing to Phase 3 trials after Phase 1, following BlueRock’s strategy.
Kang Se-il CEO stated “BlueRock conducted its Phase 1 trial with 12 patients and recently announced plans to move directly to Phase 3 after discussions with the FDA. Since TED-A9 has the same patient count and even higher efficacy, we also see a strong possibility of advancing to Phase 3.”
Additionally, S-Biomedics is expected to benefit from the Advanced Regenerative Bio Act in Korea. This law facilitates clinical trials for cell and gene therapies targeting severe, rare, and intractable diseases, allowing companies to charge patients for treatment. Since TED-A9 falls into this category, S-Biomedics could gain regulatory advantages.
Lunit Plunges Despite Major Saudi ContractOn the other hand, Lunit saw its stock plummet despite announcing a major AI imaging supply contract with Saudi Arabia. The stock dropped 9.35%(5,600 won) to close at 54,300 KRW, marking a four-day losing streak. Since February 27, when it was at 63,800 won, the stock has fallen to the 50,000 won range.
The decline was unexpected, as Lunit announced a deal with Saudi Arabia’s largest private medical group, Sulaiman Al-Habib Medical Group(HMG), to supply its AI-powered chest X-ray analysis solution, ‘Lunit INSIGHT CXR.’
HMG’s influence extends beyond Saudi Arabia to the UAE and Bahrain, and it operates over 20 medical facilities. Under this contract, Lunit anticipates analyzing over one million chest X-ray images over three years, potentially generating significant revenue.
The company has not disclosed contract details due to a non-disclosure agreement(NDA) but noted that revenue could be based either on a per-analysis pricing model or a fixed annual fee. However, investors reacted negatively despite the positive news.
Lunit expressed disappointment stating “The Saudi contract is a significant milestone for us. While there’s no apparent bad news, the recent stock decline is unfortunate. However, given Saudi Arabia’s strong regional influence, we anticipate further expansion into the Middle Eastern market.”



![QuantaMatrix, Telcon RF Rally on Gov’t Project, U.S. Supply Deal[K-Bio Pulse]](https://image.edaily.co.kr/images/vision/files/NP/S/2025/07/PS25071500182b.jpg)

![[르포] 안드로이드 탄생한 보스턴CIC가보니…"K-바이오, 빅딜 마중물"](https://image.edaily.co.kr/images/vision/files/NP/S/2025/07/PS25071401146b.jpg)
![이승호 데일리파트너스 대표 “하반기 바이오 전망 밝다, 다중항체 주목”[바이오 VC 집중조명]①](https://image.edaily.co.kr/images/vision/files/NP/S/2025/07/PS25071500005b.jpg)


