Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[Lim Jeong-yeo, Edaily Reporter] On July 31, shares of HK inno.N, Cellbion, and Rokit Healthcare gained ground in the Korean pharmaceutical, biotech, and medical device sectors. HK inno.N met 500 billion won revenue threshold for the first time for H1 earnings. Cellbion gained traction in anticipation of its prostate cancer treatment’s Phase 2 clinical data readout, estimated to take place this month. Rokit Healthcare drew interest after signaling plans to acquire a company using funds raised through recently issued convertible bonds.
 | | HK inno.N chart(Cred=KG Zeroin MP Doctor) |
|
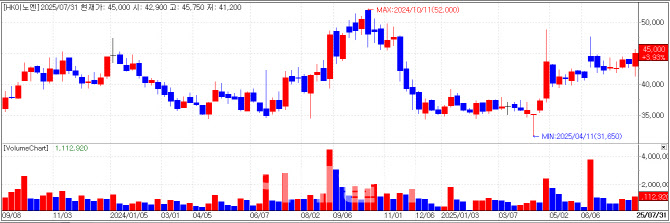
HK inno.N: Earnings, Comirnaty Contract, and U.S. Phase 3 Trial Drive Momentum HK inno.N closed at 45,000KRW on Thursday, up 6.76% (2,850 KRW) from the previous session. The rally followed the release of second-quarter results showing 263 billion KRW in revenue, a 20% increase year-on-year. This brought first-half cumulative revenue to 510 billion KRW, an 18% increase over the same period last year, marking the company’s first time surpassing 500 billion KRW threshold in H1 sales.
Investor optimism was further bolstered by progress on K-CAB, HK inno.N’s gastroesophageal reflux disease treatment, which is nearing the completion of its U.S. Phase 3 maintenance trial. The company also secured a domestic supply contract for COVID-19 vaccine Comirnaty with Pfizer last year and on July 29 was selected as the National Immunization Program(NIP) distributor. The contract covers 3.281 million doses with a total value of 213.9 billion KRW. Notably, COVID-19 vaccines were included in Korea’s NIP for the first time this year.
An HK inno.N representative said, “Implementation of the Comirnaty NIP contract is imminent, and the U.S. Phase 3 maintenance trial for K-CAB is also expected to wrap up in Q3.”
 | | Cellbion chart(Cred=KG Zeroin MP Doctor) |
|
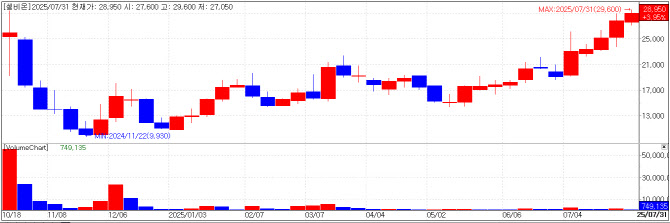
Cellbion Set to Reveal Phase 2 Data for Prostate Cancer Drug Cellbion closed at 29,050 KRW on Thursday, up 4.68% (1,300 KRW) from the day before. Shares gained on anticipation that Phase 2 trial results for 177Lu-DGUL, a radiopharmaceutical for metastatic castration-resistant prostate cancer(mCRPC), will be released in August.
Having listed on KOSDAQ last October, Cellbion is among the most advanced R&D players in Korea’s radiopharmaceutical space. 177Lu-DGUL targets the third-line treatment market for patients with mCRPC who have failed all existing therapies.
Cellbion plans to use the Phase 2 data to pursue conditional approval within the country. The company aims to launch the product in the first half of next year and generate approximately 20 billion KRW in revenue in the first year of launch. These projections were previously shared via Edaily’s premium content platform.
 | | Rokit Healthcare chart(Cred=KG Zeroin MP Doctor) |
|
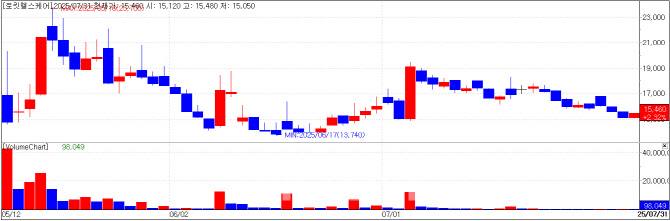
Rockit Healthcare Gains on Foreign Investment in CB Rockit Healthcare ended the day up 2.32% (350 KRW), closing at 15,460 KRW. The uptick is believed to be influenced by a PharmEdaily Premium article published that day.
Just three months after raising 17 billion KRW through a KOSDAQ IPO in May, Rockit issued 30 billion KRW worth of convertible bonds. The funds are earmarked for 22 billion KRW in operating capital and 8 billion KRW for strategic acqusition.
In an interview with Edaily, Lee Yong-gyu, CFO of Rokit Healthcare, stated that the funds would support clinical work beyond diabetic foot ulcers, including cartilage and kidney regeneration. He added, “We’re planning to acquire a company worth around 10 billion KRW to create strategic synergy.”
Among CB investors, Oasis Investment, a foreign fund, stood out by contributing 8 billion KRW (roughly 30% of the issuance). NH Investment & Securities and Korea Investment & Securities also participated, investing 7.2 billion KRW and 2 billion KRW respectively.





![HK inno.N Rises on Strong Earnings; Eyes on Cellbion Ahead of Clinical Readout[K-Bio Pulse]](https://image.edaily.co.kr/images/vision/files/NP/S/2025/08/PS25080100497b.jpg)





