Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[NA Eun-kyung, Edaily Reporter] Shares of Orum Therapeutics rebounded above their IPO price Wednesday, recovering from a sharp post-trial drop last month as investor concerns over overhang risks eased. Meanwhile, CytoGen soared to its daily upper limit after reporting record quarterly earnings, and newcomer Rokit Healthcare also hit its limit-up. MedPacto rallied on hopes surrounding its pancreatic cancer drug candidate.
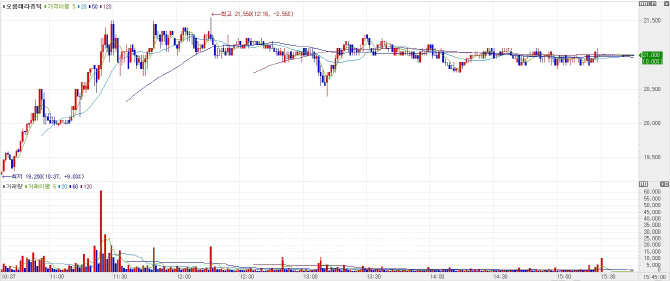
Orum Climbs Despite Lock-Up Expiry, Driven by Pipeline HopesOrum Therapeutics closed at 21,000 won on Wednesday, up 11.05% from the previous session. The rebound comes about two weeks after the company voluntarily halted its Phase 1 trial of ORM5029, which had sent shares tumbling to the 17,000-won range. The stock now sits above its IPO price of 20,000 won.
 | | Recent stock performance of Orum Therapeutic (Source: KG Zeroin MP Doctor) |
|
On Tuesday, 7.5% of Orum’s total shares became eligible for trading following the expiration of a lock-up agreement. Remarkably, the stock rose despite the added supply. A similar rally occurred in March when 28.9% of shares were unlocked, prompting some investors to joke that “Orum goes up every time lock-ups expire.”
Orum, which debuted on KOSDAQ in February via a tech-based IPO, still has 25.5% of shares under lock-up until either six months or one year post-listing. CFO Jung In-tae explained that Korean Exchange officials suggested some lock-up periods be extended to a year, and Orum’s early venture capital investors were confident enough in the company to accept the longer timeline.
Following the discontinuation of ORM5029, Orum is now accelerating development of ORM1153, an antibody-drug conjugate(DAC) built on its TPDsquare protein degradation platform. The company says ORM1153, although using the same payload as ORM5029, has a different antibody and linker, reducing the risk of similar side effects.
“Unlike conventional chemotherapy-based treatments, ORM1153 works through a different mechanism,” said Jung. “It could provide an option for relapsed patients who’ve already undergone other therapies.” Orum aims to submit an IND application for ORM1153 by the end of next year.
CytoGen Hits Daily Limit on Record-Breaking Q1 ResultsLiquid biopsy firm CytoGen saw its shares climb 29.94% to close at 4,405 won, hitting the daily price ceiling. The rally came after the company reported record-breaking quarterly results.
First-quarter consolidated revenue reached 5.6 billion won, marking a 606% increase from 800 million won in the same period last year. CytoGen expects this momentum to continue through year-end.
“Our annual revenue reached 10.9 billion won last year, and we’ve now set internal targets to surpass that in 2025,” a company official told Edaily. “We’re especially focused on expanding in the U.S. and Japan.”
In the U.S., the company is boosting sales via its CLIA-certified lab subsidiary ExperTox. Q1 U.S. sales have already risen year-over-year, and meaningful contributions are expected from the second half onward. The company is advancing talks to supply its circulating tumor cell (CTC) platforms to major U.S. cancer centers and hospitals.
In Japan, CytoGen was recently selected for the country’s largest cancer research initiative, achieving its first local revenue since founding its Japanese subsidiary last year. The company expects stronger sales in Japan starting in the second half.
MedPacto Rises on MP010 Hopes Ahead of Global ConferencesMedPacto shares rose 12.69% to close at 3,685 won, fueled by investor anticipation ahead of major global biotech events. The company plans to present its pancreatic cancer candidate MP010 later this month at the American Society of Clinical Oncology (ASCO) meeting and at BIO USA in June.
“We’ll be sharing our clinical entry and early license-out strategies for MP010,” a company representative said. “The patent is set to be published later this month, allowing us to highlight its key features in detail.”
MP010 is a dual fusion protein that disrupts the tumor microenvironment (TME), allowing immune cells to better attack cancer cells. MedPacto plans to complete toxicity studies by the end of this year and file for a Phase 1 clinical trial in 2025. The company is also considering out-licensing the candidate during or shortly after the Phase 1 stage.



![2% Royalty Shock at Alteogen Ripples Through Korean Biotech[K-Bio Pulse]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/01/PS26012200181b.jpg)



!['2% 로열티'가 무너뜨린 신뢰…알테오젠發 바이오株 동반 하락[바이오맥짚기]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/01/PS26012201091b.jpg)


