Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[Song Young-doo, Edaily Reporter] Orum Therapeutics shares plunged after the company announced the voluntary termination of a key clinical trial. The news triggered a broader decline across South Korean biotech and pharmaceutical stocks. In contrast, Vaxcell-Bio saw a sharp rally after receiving the country’s first regulatory approval for an autologous bone marrow-derived cell processing facility. InventageLab also posted gains amid speculation about potential license-out opportunities for its oral obesity treatment platform.
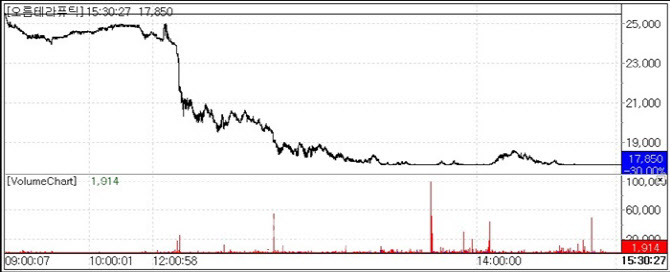 | | Orum Therapeutics Stock Trend (Source: KG Zeroin MPDOCTOR) |
|
According to KG Zeroin’s MPDOCTOR platform(formerly MarketPoint) on April 28, Orum Therapeutics shares weakened from the opening bell and sharply dropped around 1:40 p.m. The stock, which had closed at KRW 25,500 the previous day, plummeted 30%(KRW 7,650) to end at KRW 17,850. The company announced the voluntary termination of its Phase 1 trial for ‘ORM-5029’ a treatment targeting HER2-expressing advanced solid tumors.
In a public disclosure, Orum explained that the decision was made based on a comprehensive assessment of the clinical safety, pharmacokinetics(PK), and pharmacodynamics(PD) data from the Phase 1 study. “This decision prioritizes patient safety and reflects our commitment to developing therapeutics with a clear risk-benefit profile” the company said. It added that the move would allow the company to strategically focus resources on next-generation pipelines based on its proprietary platform technologies.
Industry analysts interpret the termination as being linked to safety concerns. Last November, Orum Therapeutics had halted patient enrollment in the U.S. Phase 1 study following a serious adverse event(SAE) resulting in a patient death due to liver failure. SAEs refer to cases involving death, life-threatening conditions, hospitalization, permanent disability, or fetal abnormalities.
At the time, experts suggested that the SAE might not necessarily derail the program but emphasized the importance of closely reviewing Orum’s investigation results. A former MFDS official noted, “Many cancer patients enrolled in trials are in critical condition, so SAEs are expected. Proper evaluation should be based on the root cause analysis.”
However, the decision to end the program implies that the toxicity risks outweighed the therapeutic benefits, according to industry consensus.
Vaxcell-Bio Soars on Cell Processing Facility Approval While most biotech stocks declined under the weight of Orum‘s news, Vaxcell-Bio bucked the trend, surging sharply. On April 28, Vaxcell-Bio shares jumped 16.72%(KRW 1,569) from KRW 9,330 to close at KRW 10,990.
The rally was attributed to the company’s announcement that it had received regulatory approval from the Ministry of Food and Drug Safety(MFDS) for an autologous bone marrow-derived cell processing facility. Analysts believe this approval could positively impact the development of its flagship NK cell therapy for liver cancer and CAR-MIL therapy for solid tumors, as well as pipeline programs targeting advanced heart failure under Korea’s Advanced Regenerative Bio Act.
A Vaxcell-Bio official commented, “We have focused primarily on developing NK cell therapies and have obtained clinical trial approvals for CAR-T therapies. Now, with this facility approval, we can expand our capabilities to autologous bone marrow-derived cell therapies.” They added, “This will expedite our clinical development process under the Advanced Regenerative Bio Act.”
After establishing GMP facilities in July 2024, Vaxcell-Bio obtained MFDS approvals for its NK cell processing facility in August and its CAR cell processing facility in December. CEO Lee Je-joong stated, “We have systematically prepared for the enactment of the Advanced Regenerative Bio Act and will now lead clinical research in the regenerative medicine sector. We also plan to explore multifaceted uses of our GMP facilities to meet growing CDMO demand.”
InventageLab Rebounds on PharmEdaily Interview Highlighting Oral Obesity Drug InventageLab also rebounded after falling earlier in the week. The company had previously reported a 24.3% bioavailability rate for its oral obesity drug candidate IVL3027 during a company IR session on April 23, marking a 73-fold improvement compared to Novo Nordisk’s oral semaglutide Rybelsus. Although the stock initially surged to the daily limit on April 24, it slipped on April 25.
However, shares rebounded after PharmEdaily published an interview with Inventage Lab CEO Kim Joo-hee on April 28 (“Inventage Lab CEO Kim Joo-hee Pioneering Infinite Expansion of Oral Obesity Platform”). Following the interview’s release, shares rose 9.70%(KRW 2,450) to close at KRW 27,700 compared to the previous session. Notably, the stock continued to surge after hours, rising 23.17% to KRW 31,100 on the alternative trading platform NextTrade(NTX).
In the interview, CEO Kim revealed, “Since the announcement of our oral obesity drug data, we’ve been receiving simultaneous inquiries from global big pharma. We have heard from Novo Nordisk’s Asia division and are scheduling additional meetings with their U.S. division as well.”
InventageLab is already collaborating with Boehringer Ingelheim on long-acting injectable therapies and is now attracting broader attention in the oral therapeutic space as well.










