Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[Shin-Min Joon, Edaily Reporter] On July 3, Korea’s biopharmaceutical stock market saw notable movements from Rokit Healthcare, AptamerSciences and GL PharmTech.
Rokit Healthcare shares surged following news that the company had successfully completed a clinical trial for AI-based regenerative therapy for skin cancer. GL PharmTech rebounded as investor sentiment improved on expectations that management efficiency would improve through the absorption of its subsidiary, GL Pharma.
AptamerSciences hit the upper price limit during intraday trading amid speculation that it may be acquired by Aribio. However, the stock plummeted later in the session after Aribio denied the acquisition rumors.
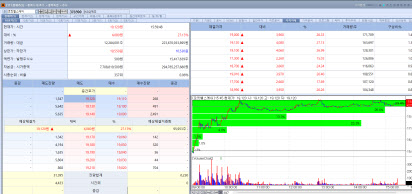 | | Rokit Healthcare stock trend on July 3. (Image=MP Doctor) |
|
Rokit Healthcare Announces Successful AI Based Skin Cancer Regenerative Therapy Trial in Japan According to MP Doctor (formerly MarketPoint), a subsidiary of KG Zeroin, shares of Rokit Healthcare surged 27.13% on the day to close at won19,120. The rally came after the company officially announced the successful completion of a clinical case in Japan using its AI powered skin regeneration platform to reconstruct skin tissue in a skin cancer patient.
At the 17th Annual Meeting of the Japanese Society for Wound Healing held in Tokyo, Rokit Healthcare presented a clinical case where normal skin tissue was fully regenerated within four weeks using only its AI based regenerative treatment platform in an elderly skin cancer patient for whom surgery or grafting had previously been impossible.
The clinical trial was conducted and presented by Dr. Hajime Matsumura President of the Japanese Society for Wound Healing and Professor at Tokyo Medical University.
He stated “Rokit’s AI regenerative technology is a game changer shifting the paradigm from conventional wound surgery to regeneration centered treatment. This cutting edge, ultra personalized AI technology is essential not only for Japan but for aging medical systems worldwide.”
The clinical results were also published in the international SCI journal Journal of Clinical Medicine.
A Rokit Healthcare representative explained “This trial involved 10 patients with skin cancer. The results showed full skin regeneration within an average of 4.2 weeks and an average patient satisfaction score of 280 out of a maximum 300 on the SCAR-Q aesthetic assessment.”
The representative added “There were no immune rejection reactions and scarring was minimal. Especially in cosmetically sensitive areas such as the nose, forehead, and cheeks, the platform significantly outperformed conventional surgery.”
He continued “A major advantage is that the treatment is also applicable to elderly patients and those with systemic illnesses who are unsuitable for conventional therapy. This marks the world’s first successful clinical application of an ultra-personalized AI driven regenerative therapy for skin cancer.”
Unlike conventional skin grafts Rokit Healthcare’s platform precisely analyzes the tissue structure of lesions using AI, designs customized regenerative tissues and implements printing and transplantation. Skin cancer is reportedly on the rise due to global aging and climate change.
Non melanoma skin cancer in particular accounts for millions of surgeries annually. According to market research firm IMARC the global market for skin cancer regenerative therapies was estimated at up to $700 million (950 billion won) in 2024 and is expected to grow to over $900 million (1.22 trillion won) by 2030.
A Rokit Healthcare representative explained “Based on these results we plan to begin full scale commercialization of our AI skin regeneration platform in major global regions including Japan, Korea, the U.S., Europe and South America starting in the second half of this year.”
The representative added “The company is also expanding the platform’s applications beyond diabetic foot ulcers to include bedsores, traumatic skin defects, severe burns, and high risk wounds”
GL PharmTech to Absorb Subsidiary GL Pharma GL PharmTech shares rose 4.10% to won 1,244 rebounding from a previous day’s decline. The increase is attributed to the company’s announcement of a merger with its subsidiary GL Pharma.
The merger will be a small scale consolidation without issuing new shares leaving GL PharmTech as the surviving entity while GL Pharma will be dissolved. The merger date is set for September 11.
Following the merger GL Pharma’s personnel licenses and facilities will all be transferred to GL PharmTech. Previously some sales and R&D operations were separated under GL Pharma. The merger is expected to unify operations, eliminate redundancies, enhance managerial efficiency and create interdepartmental synergy.
Since WScience became its largest shareholder in March last year GL PharmTech has focused on normalizing management and corporate restructuring. Several executives formerly with Hanmi Pharmaceutical have joined the leadership helping to boost the company’s value.
The company turned profitable starting in Q3 2024, reflecting its restructuring efforts. The upward trend has continued into 2025. In Q1 2025 the company recorded 7.8 billion won in revenue, a 34.4% increase year over year, with 100 million won in operating profit marking a return to profitability.
GL PharmTech representative explained “Through the merger between GL PharmTech, a drug R&D specialist and GL Pharma which owns a domestic KGMP certified manufacturing facility, we are now eligible to apply for designation as an Innovative Pharmaceutical Company.”
GL PharmTech representative added “The designation would provide benefits such as preferential drug pricing, tax incentives, and regulatory support for expanded R&D, manufacturing, and marketing efforts.”
Aptamer Sciences Falls on Denied Acquisition Rumors by Aribio Shares of Aptamer Sciences fell 4.85% to won 1196. The stock initially surged to the daily upper limit on speculation that it might be acquired by drug developer Aribio but plummeted after Aribio officially denied the rumors.
Aptamer Sciences hit the 29.99% intraday ceiling right after the market opened but dropped once Aribio publicly dismissed the acquisition claims. Aptamer Sciences was listed on the KOSDAQ in 2020 through the special technology listing track. The company develops diagnostics and biopharmaceuticals including cancer therapeutics and targeted therapies based on aptamers, synthetic DNA fragments that bind to specific proteins or cells and are viewed as potential antibody alternatives.
The company has been exploring various strategic investments and co development deals since early 2025. This led to reports that Aptamer Sciences was up for sale and that Aribio was a strong candidate for acquisition. However, Aribio denied any such intention.
In a statement Aribio said “The letter of intent for acquisition referenced in the report was not drafted by us but by an affiliated agency.”
The company added “There seems to have been some misunderstanding or misinterpretation regarding the transaction. While Aribio internally reviewed the proposal we determined it is not viable at this time and have no current plans to pursue the acquisition.”
Aribio also clarified “Our ongoing merger with Solux is proceeding as planned and we intend to submit the amended securities report by the July 8 deadline.”