Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[Seungkwon Kim, Edaily Reporter] Stocks of South Korean pharmaceutical, biotech, and healthcare companies surged on Thursday, buoyed by major licensing deals and growing investor expectations for clinical trial results.
NIBEC shares continued to soar for a second consecutive day, following the announcement of a major licensing agreement. Meanwhile, Aptabio and Mezzion stocks rallied as investors eyed promising clinical trial data.
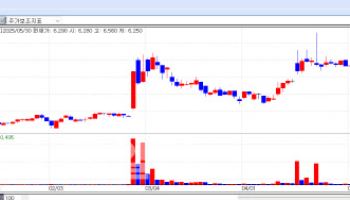
NIBEC Secures Massive Licensing Deal, Shares Surge Shares of NIBEC closed at 55,350 won, up 22% from the previous day, after hitting the daily limit up for two consecutive days. Its market capitalization jumped from 260 billion won before the announcement to 390 billion won.
The company revealed that it had signed a licensing agreement with a U.S.-based biotech firm for its key pipeline NP-201, a peptide-based therapy targeting fibrosis. Unlike traditional treatments, NP-201 promotes regeneration of damaged tissue through a novel mechanism.
 | | NIBEC Daily Stock Price Trend (Data: KG Zeroin) |
|
Having completed a successful Phase 1 trial in Australia, NIBEC is preparing for Phase 2 trials. The U.S. partner reportedly has strong R&D and commercialization capabilities in idiopathic pulmonary fibrosis (IPF) and pulmonary arterial hypertension (PAH).
The licensing deal is valued at up to $435 million, more than double NIBEC’s market capitalization. The initial payment amounts to $8 million, with additional royalties of 4% on net sales. NIBEC will also handle manufacturing, leveraging its cGMP-certified facilities.
In addition to NP-201, NIBEC boasts a promising pipeline including anti-aging, KRAS inhibitor oncology drugs, and advanced drug delivery systems, increasing expectations for future licensing deals.
A NIBEC spokesperson stated, “There are no additional details we can disclose beyond the licensing announcement.”
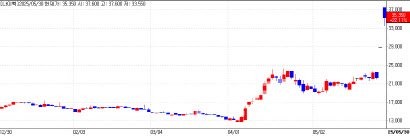
Mezzion’s Shares Rise Amid FDA Approval Anticipation Shares of Mezzion, a company developing therapies for rare diseases, closed 10% higher at 40,100 won. The rally was fueled by optimism surrounding its pending FDA approval for a Fontan-associated treatment.
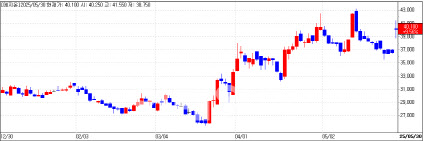 | | Mezzion Daily Stock Price Trend (Data: KG Zeroin) |
|
Mezzion recently announced the inclusion of Fontan-related complications in the WHO’s International Classification of Diseases (ICD-11), marking a milestone for clearer diagnosis and treatment of this complex condition. Fontan surgery, performed on patients with congenital heart defects, often leads to severe complications affecting quality of life.
Industry experts believe this classification will streamline clinical trial designs and facilitate regulatory approvals. Mezzion is conducting additional clinical trials (FUEL-2) of its Udenafil drug to boost exercise capacity in Fontan patients. It is also expanding indications for autosomal dominant polycystic kidney disease (ADPKD).
Aptabio’s Stock Soars on Immunotherapy Prospects Aptabio (KQ:293780) shares closed nearly 8% higher at 8,590 won, amid optimism over its new solid tumor therapy. The company’s presentation at the 2025 American Association for Cancer Research (AACR) annual meeting in Chicago garnered attention for revealing new insights into the role of NOX enzymes in cancer-associated fibroblasts (CAFs) and M2 macrophages.
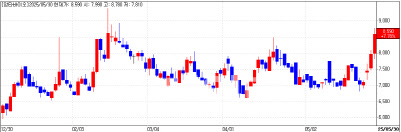 | | Aptabio Daily Stock Price Trend (Data: KG Zeroin) |
|
Aptabio’s APX-343A, a selective NOX inhibitor, is designed for solid tumors resistant to immune checkpoint inhibitors, a group representing up to 80% of patients. The company signed a collaboration and supply agreement with Merck (MSD) last year to combine APX-343A with Keytruda, the world’s top-selling drug with $25 billion in annual sales.
In addition to partnerships, Aptabio has expanded its global patent portfolio by securing patents in Japan and Mexico for APX-343A, further solidifying its path to commercialization.





![NIBEC’s Mega Licensing Deal Sends second Shares Soaring[K-Bio Pulse]](https://image.edaily.co.kr/images/vision/files/NP/S/2025/06/PS25060100048b.jpg)


![‘올해 실적 좋아요’…큐렉소, 올해 로봇 판매 100대 이상[인베스트 바이오]](https://image.edaily.co.kr/images/vision/files/NP/S/2025/06/PS25060100331b.jpg)