Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
[Kim Jiwan, Edaily Reporter]On the 7th, Korea’s biopharmaceutical sector, TiumBio and Biosolution Surge on Clinical Successes.
Meanwhile, OrientBio hit the upper limit on news that a key trial involving opposition leader Lee Jae-myung was postponed until after the presidential election.
TiumBio: Merigolix Succeeds in Phase 2 According to MP Doctor by KG Zeroin on May 7, TiumBio Co., Ltd. rose 10.10 percent to close at KRW 5,670. The company announced that its investigational oral GnRH antagonist Merigolix (TU2670) successfully met the primary endpoint of reducing heavy menstrual bleeding in a Phase 2 clinical trial for uterine fibroid treatment. The trial, conducted by its domestic partner Daewon Pharmaceutical Co., Ltd., demonstrated statistically significant improvements across all dosage groups compared to placebo.
Unlike existing GnRH agonists that are administered via injection and cause an initial hormone surge, Merigolix offers oral administration and provides a faster onset of action without hormonal flare, offering greater convenience and tolerability for patients. In the trial, 71 patients were randomly assigned to high-, medium-, low-dose, and placebo groups, and were treated once daily for 12 weeks, followed by a 12-week observation period. All Merigolix treatment groups showed statistically significant improvements in heavy menstrual bleeding compared to placebo.
In addition to the primary endpoint, Merigolix showed strong performance in key secondary endpoints including fibroid volume reduction, improvement in hemoglobin levels, and relief of pelvic pain. The drug also demonstrated good safety and tolerability across all dosage levels. TiumBio CEO Hoon-Taek Kim stated that Merigolix has now demonstrated excellent therapeutic effect in both endometriosis and uterine fibroids, adding that the successful completion of the challenging Phase 2 trial proves the drug’s efficacy and safety, thereby increasing the likelihood of regulatory approval and global licensing opportunities.
According to Global Market Insights, the global uterine fibroid treatment market is expected to grow from KRW 2.5 trillion in 2022 to approximately KRW 6.6 trillion by 2032, with an annual growth rate of around 10 percent.
Biosolution: CartiLife Shows Positive Results in FDA Phase 2 Shares of Biosolution Co., Ltd. climbed 11.32 percent to KRW 30,000 after the company announced positive results from its FDA Phase 2 trial of CartiLife, an autologous chondrocyte implantation (ACI) cell therapy for knee cartilage regeneration. The trial, conducted from November 14, 2019, to December 16, 2024, included 20 patients with traumatic or degenerative cartilage defects in the knee and evaluated both the safety and efficacy of the CartiLife treatment.
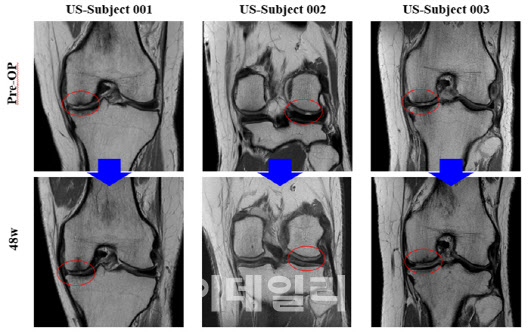 | | Preoperative and postoperative MRI images of a U.S. patient show that the cartilage defect was filled after 48 weeks of treatment. (Provided by Biosolution) |
|
At 48 weeks, patients showed an average increase of 34.7 points from baseline in the KOOS subscore for sports and recreational activities in both the ITT and PP analysis populations. Furthermore, more than half of the patients, specifically 10 out of 19, achieved the highest defect fill score of 20 points, indicating that 100 to 150 percent of the cartilage defect volume was regenerated.
Based on the favorable results, Biosolution plans to explore opportunities for entering the U.S. market. The company had previously announced on April 30 that CartiLife received full marketing approval from Korea’s Ministry of Food and Drug Safety (MFDS), following successful completion of a confirmatory Phase 3 trial and the upgrade from conditional approval granted in 2019.
In South Korea, the cartilage defect and osteoarthritis treatment market is valued at approximately KRW 400 billion and includes around 4 million patients. The global market is projected to grow from USD 7.3 billion in 2019 to USD 11 billion in 2025, with the U.S. market estimated at over KRW 5 trillion and an affected population of approximately 40 million.
OrientBio Surges on Delay of Lee Jae-myung Trial OrientBio Inc. hit the upper trading limit after news that Democratic Party presidential candidate Lee Jae-myung’s retrial on charges of violating the Public Official Election Act had been postponed until after the presidential election.
The court rescheduled the initial trial date from May 15 to June 18, which places it after the June 3 election.
OrientBio has been classified as a “Lee Jae-myung theme stock” due to Lee’s reported past employment at its affiliate company, Orient Watch, during his youth. However, there is no confirmed business connection between Lee and OrientBio.
 | | Lee Jae-myung, presidential candidate of the Democratic Party of Korea, pauses to drink water while greeting citizens at Imsil Market in Imsil County, North Jeolla Province, during his “Neighborhood Listening Tour: Cross-Country Edition” on May 7. (Photo = Yonhap News) |
|







![[韓 AI신약개발 진단]① K-신약 인공지능, 美와 격차 벌어지는 까닭](https://image.edaily.co.kr/images/vision/files/NP/S/2025/05/PS25050900885b.jpg)

![FDA, 외국기업 의약품 제조시설 불시 검사 확대[제약·바이오 해외토픽]](https://image.edaily.co.kr/images/vision/files/NP/S/2025/05/PS25051000232b.jpg)

