[Seok Ji-hoen, Edaily Reporter] Biosolution, a leading advanced biopharmaceutical company, announced that it has received the final Clinical Study Report (CSR) for the Phase 3 clinical trial of CartiLife, its next-generation knee cartilage regeneration cell therapy, conducted in South Korea.
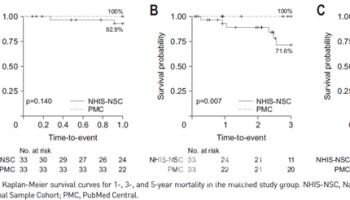
The results include 96-week efficacy data, following the topline findings from the 48-week primary efficacy analysis released in April 2024. The primary efficacy endpoint, the MOCART score, demonstrated a statistically significant improvement of over 11 points compared to the control group, confirming the treatment’s effectiveness.
The clinical trial, conducted at 19 sites across the country, enrolled 104 participants from May 2020. The results showed that CartiLife outperformed the active control group (microfracture surgery) in multiple key measures, including the MOCART score (cartilage regeneration), KOOS total score, cartilage defect repair, and integration of regenerated cartilage with surrounding tissues. At week 96, the MOCART score for the CartiLife group was 47.98, compared to 36.59 for the microfracture surgery group, demonstrating a statistically significant efficacy advantage of over 11 points. The p-value was 0.0131, well below the significance threshold of 0.05. Notably, CartiLife also showed cartilage regeneration benefits in patients aged 60 and older, those with mobility defects, and those with osteoarthritis. Additionally, pain reduction and functional improvement were sustained through week 96.
A company representative stated, “In the 48-week data released last year, the difference between the treatment and control groups was 9 points. Now, at 96 weeks, the gap has widened to 11 points, reaffirming the superiority of CartiLife over time.” The company plans to present detailed findings at the American Academy of Orthopaedic Surgeons (AAOS) on March 10 and the Osteoarthritis Research Society International (OARSI) on April 24.
One of the most significant findings was the structural integrity and signal intensity of regenerated tissue, which closely resembled native hyaline cartilage rather than fibrocartilage, a common limitation of microfracture-based cartilage regeneration techniques. Unlike microfracture surgery, which primarily regenerates fibrocartilage (similar to intervertebral disc cartilage), CartiLife successfully restored hyaline cartilage, demonstrating superior cartilage quality.
Biosolution plans to submit the final report and supporting documents to South Korea’s Ministry of Food and Drug Safety (MFDS), aiming for regulatory approval. Given the statistical superiority demonstrated in the Phase 3 trial, the company expects a higher likelihood of approval. Meanwhile, the U.S. Phase 2 clinical trial for CartiLife has completed its final patient follow-up, with final analysis now underway.
“We are highly encouraged to see the excellent efficacy and safety results confirmed in the final clinical study report,” said Lee Jung-sun, CEO of Biosolution. “The slight decline in absolute MOCART scores at 96 weeks compared to 48 weeks was due to some cases of cartilage overgrowth in a subset of patients. However, this can be mitigated by optimizing the dosage during transplantation. While many cartilage regeneration procedures struggle with inadequate growth, CartiLife’s strong regenerative capacity is a clear advantage.”






![트럼프 행정부, 中의약품 허가 제한 행정명령 저울질[제약·바이오 해외토픽]](https://image.edaily.co.kr/images/vision/files/NP/S/2025/09/PS25091300149b.jpg)